Abstract:
Supplementation with antioxidants is widely considered a promising approach for addressing male subfertility. Nonetheless, the comparative effectiveness of various antioxidant agents on semen quality parameters is not well established. This network meta-analysis (NMA) aimed to compare the effects of different antioxidant supplements on semen parameters in men with subfertility. A systematic search was conducted across PubMed, Embase, and the Cochrane Library for randomized controlled trials (RCTs) from database inception through January 31, 2021. The analysis included eight antioxidants—folic acid, zinc, vitamin E, carnitine, selenium, coenzyme Q10 (CoQ10), N-acetylcysteine, and vitamin C—along with placebo controls. For each semen outcome (concentration, motility, and morphology), a Bayesian NMA with random-effects modeling was employed, and the surface under the cumulative ranking curves (SUCRAs) was calculated to assess relative efficacy. Eighteen RCTs involving 1790 subfertile men were analyzed. CoQ10 showed a statistically significant improvement in sperm concentration (mean difference [MD] = 5.95; 95% confidence interval [CI]: 0.05, 10.79) versus placebo and ranked highest in efficacy (SUCRA: 79.4%). Regarding sperm motility, both carnitine (MD = 12.43; 95% CI: 4.07, 20.26) and CoQ10 (MD = 7.33; 95% CI: 0.35, 14.17) demonstrated significant benefits, with carnitine ranking most effective (SUCRA: 88.7%). Although vitamin C showed the highest SUCRA score for sperm morphology (93.6%), its effect did not reach statistical significance (MD = 7.73; 95% CI: –0.94, 16.33). In conclusion, CoQ10 and carnitine appear to be the most effective antioxidant interventions for improving sperm concentration and motility, respectively, in men with subfertility.
Keywords: subfertility, antioxidants, sperm parameters, coenzyme Q10, carnitine, network meta-analysis
Introduction
Subfertility is a broad term encompassing all degrees of reduced fertility in couples experiencing difficulty conceiving despite regular unprotected intercourse (1). Globally, approximately 15% of couples are affected by this condition, with male-related factors accounting for nearly 50% of cases (2). Semen analysis remains the primary method for evaluating male fertility. Deviations in semen quality are assessed through both quantitative (e.g., azoospermia, oligospermia) and qualitative (e.g., asthenozoospermia, teratozoospermia) measures (3). A recent global meta-analysis revealed a significant decline in human semen quality over the past four decades (4). Consequently, identifying effective strategies to enhance semen quality in subfertile men has become a significant public health focus.
Spermatogenesis involves a highly regulated sequence of molecular and biochemical events. As such, the specific underlying mechanisms of subfertility in men remain largely undefined (5). One common mechanism implicated in 30–80% of subfertile cases is oxidative stress caused by excessive reactive oxygen species (ROS) (6). These oxygen-based molecules, generated through normal cellular processes, are highly reactive and unstable. While ROS serve essential signaling roles and contribute to sperm function and fertilization (7), their overproduction can surpass the body’s antioxidant defenses. This imbalance leads to oxidative stress, which disrupts sperm function, development, and DNA integrity (8). Additionally, studies have noted that in oligospermic men, sperm often reside longer in the epididymis, increasing susceptibility to ROS-induced damage (9). Given the substantial financial burden of assisted reproductive techniques, antioxidant supplementation has gained traction as a cost-effective and accessible method to reduce oxidative stress and improve sperm quality. Their oral availability, favorable safety profile, and high bioavailability further support their widespread use (10).
Numerous studies over recent decades have underscored the protective roles of various antioxidants—including carnitine, vitamin E, zinc, and folate—on sperm health (7, 10–12). Supplementing with antioxidants has been shown to enhance semen quality in subfertile men and may increase the likelihood of spontaneous conception while lowering the financial demands associated with achieving pregnancy (13). A Cochrane meta-analysis, most recently updated in 2019, reviewed antioxidant treatments for subfertility and concluded that such therapies can improve semen parameters and may also raise clinical pregnancy rates (14). While this supports the efficacy of antioxidants, the analysis did not differentiate among specific antioxidant types, largely due to limited head-to-head comparisons. To address this gap, network meta-analysis (NMA) serves as a powerful method of evidence synthesis, integrating both direct and indirect comparisons into a single statistical model when direct data are sparse (15). Accordingly, this systematic review and NMA aim to assess and compare the effects of various antioxidants on semen quality parameters in subfertile men.
Methods
This network meta-analysis (NMA) was registered in the International Prospective Register of Systematic Reviews under the identifier CRD42021234266. The results were reported following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension specific to NMAs (16).
Search Strategy
A comprehensive literature search was conducted in the PubMed, Embase, and Cochrane Library databases from inception until 31 January 2021. The search strategy was based on the antioxidant agents identified in a prior Cochrane review on male subfertility (14), including nine antioxidant substances with known activity: arginine, folic acid, zinc, vitamin E, carnitine, selenium, coenzyme Q10 (CoQ10), N-acetylcysteine, and vitamin C. No restrictions on language were applied during the database searches. Details of the full search methodology are included in Supplemental Appendix 1. Two reviewers (LS and JZ) independently screened the retrieved articles for eligibility, and any disagreements were resolved through discussion with a third reviewer (Y-zJ).
Study Selection
To be included, studies had to meet the following criteria:
- randomized controlled trials (RCTs) using either parallel or crossover designs, although for crossover trials, only first-phase data were considered;
- study populations consisting of subfertile men exhibiting abnormal semen profiles, including oligozoospermia, asthenozoospermia, or teratozoospermia;
- trials involving a single antioxidant intervention compared against either placebo or another antioxidant;
- studies reporting on at least one of the following outcomes: sperm concentration, sperm motility, or sperm morphology.
Studies were excluded if fertility-enhancing cointerventions were administered selectively to certain groups within the study, thereby compromising comparison validity.
Data Extraction
Data extraction was conducted independently by two reviewers (LS and JZ), who collected the following information from each included study: first author’s name, publication year, country of origin, total sample size, average participant age, mean BMI, details of interventions (including antioxidant type or placebo), treatment duration, and post-treatment outcomes for sperm concentration, motility, and morphology, along with associated standard deviations (SDs).
Risk of Bias Assessment
The methodological quality of each included RCT was appraised by LS and JZ independently using the Cochrane Collaboration’s tool for assessing risk of bias (17). The following domains were evaluated: method of random sequence generation, allocation concealment, blinding procedures, completeness of outcome data, selective outcome reporting, and other potential sources of bias. Studies were categorized based on the overall risk of bias:
- high risk—if any domain was rated high;
- low risk—if at least three domains were rated low with none high;
- moderate or unclear risk—if they did not fall into the previous categories (18).
Data Synthesis and Statistical Analysis
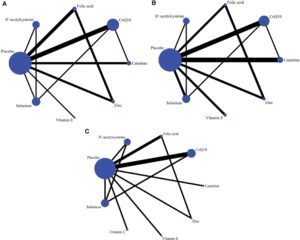
Network plots were created for each outcome variable to visually represent the direct comparisons among the interventions (19). In these plots, the node size reflects the number of studies evaluating each intervention, while line thickness represents the frequency of comparisons.
A pairwise meta-analysis using a random-effects model was performed using Stata 15 (StataCorp, College Station, TX) to estimate pooled effects, as the random-effects approach offers more conservative estimates (20). Mean differences (MDs) with 95% confidence intervals (CIs) were calculated for all continuous outcomes. If multiple arms used the same antioxidant in different dosages or formats, pooled means and SDs were computed per the Cochrane Handbook’s recommendations (21). In instances where studies reported medians and interquartile ranges (IQRs) rather than means and SDs, conversions were carried out using the methods proposed by Luo et al. (22) and Wan et al. (23), respectively.
A Bayesian NMA was conducted using the ADDIS software version 1.16.5 (Drug Information Systems, Groningen, Netherlands) to incorporate both direct and indirect comparisons under a random-effects framework with noninformative priors (24). Markov chain Monte Carlo (MCMC) simulation was utilized with four independent chains, an initial value of 2.5, step size of 10, burn-in of 20,000 iterations, and 50,000 iterations for posterior simulation. Convergence of the Bayesian models was evaluated using the Brooks–Gelman–Rubin diagnostic, with the potential scale reduction factor (PSRF) as the key indicator. Values approaching 1 indicated good convergence.
To assess and compare the relative effectiveness of all antioxidant interventions, ranking probabilities were calculated using ADDIS, and results were summarized using surface under the cumulative ranking curves (SUCRAs). To test for the assumption of transitivity—a key condition for NMA validity—differences in key modifiers such as age and treatment duration were examined across studies. Network inconsistency, or disagreement between direct and indirect evidence, was evaluated using node-splitting techniques (25).
Sensitivity analyses were performed by excluding studies that relied on imputed values or were rated as high risk of bias. Publication bias and small-study effects were examined via comparison-adjusted funnel plots to detect asymmetry for each outcome (19).
Results
From the initial pool of 1,992 identified records, 48 full-text articles were assessed for eligibility. Ultimately, 18 randomized controlled trials (RCTs) met the inclusion criteria and were incorporated into the systematic review and network meta-analysis (NMA), encompassing a total of 1,790 subfertile male participants (26–43) (Figure 1). These studies evaluated eight different antioxidants: folic acid, zinc, vitamin E, carnitine, selenium, coenzyme Q10 (CoQ10), N-acetylcysteine, and vitamin C. Arginine was excluded from the analysis due to the absence of qualifying trials. Only five RCTs (26, 31, 32, 39, 42) conducted head-to-head comparisons between two or more antioxidant agents.
FIGURE 1 Flow diagram of the study selection process.
The trials were conducted across various countries. Specifically, eight trials originated from Iran (30–34, 36, 38, 43); three from Italy (35, 40, 41); and one each from The Netherlands (26), Saudi Arabia (27), the United States (28), the United Kingdom (29), Brazil (37), China (39), and Iraq (42). Treatment durations ranged from 12 to 26 weeks. Participants had a mean age ranging from 25.54 to 36.24 years and were all diagnosed with abnormal semen profiles.
The outcome sperm concentration was assessed in 13 studies (26, 30–33, 35–37, 39–43); sperm motility in 16 studies (26–36, 39–43); and sperm morphology in 10 studies (26, 30, 31, 33, 34, 36–38, 42, 43).
Network Diagrams
The network plots for all three outcomes are shown in Figure 2. Across all comparisons, the majority involved comparisons between individual antioxidants and placebo. Figure 2A depicts the network of 13 RCTs reporting sperm concentration, covering seven antioxidants and the placebo. Figure 2B shows the sperm motility network, including data from 16 RCTs. Figure 2C illustrates the network for sperm morphology, incorporating 10 RCTs and involving eight antioxidants alongside placebo.
Risk of Bias
Of the 18 trials, 11 explicitly described methods for generating random sequences, although all mentioned randomization. Allocation concealment was reported in nine trials, while the remaining did not provide sufficient detail. Regarding blinding, 15 studies were classified as having low risk for blinding of participants and personnel, and 8 had low risk for blinding of outcome assessment. One study was deemed high risk due to inadequate blinding procedures. Twelve studies reported complete outcome data and were rated low risk, while two were flagged for high risk of incomplete data. With respect to selective reporting, three trials were deemed low risk and one was identified as high risk. Overall, three studies (27, 30, 37) were judged to have high risk of bias, ten studies (26, 28, 29, 31–33, 36, 38, 40, 43) had low risk, and five (34, 35, 39, 41, 42) were categorized as having unclear or moderate risk.
Transitivity, an assumption necessary for valid indirect comparisons in NMA, was evaluated. Differences in participant age were minimal across studies. Treatment durations were considered sufficiently homogeneous, with all trials lasting at least 74 days, corresponding to the duration of one full spermatogenesis cycle (44).
Outcome Analysis
Sperm Concentration
In the pairwise random-effects meta-analysis, three antioxidants—CoQ10, selenium, and N-acetylcysteine—produced statistically significant improvements in sperm concentration when compared with placebo (P < 0.05).
The NMA further supported these findings. Among the tested antioxidants, CoQ10 showed a significant benefit, increasing sperm concentration with a mean difference (MD) of 5.95 (95% CI: 0.05, 10.79) versus placebo. None of the other antioxidants demonstrated statistically significant advantages over placebo, including folic acid, zinc, vitamin E, carnitine, selenium, N-acetylcysteine, and vitamin C. Ranking analysis using the surface under the cumulative ranking curve (SUCRA) placed CoQ10 at the top (SUCRA: 79.4%) for improving sperm concentration, followed by carnitine (65.4%) and folic acid (56.3%) (Supplemental Figure 4).
Sperm Motility
In the direct pairwise meta-analysis, carnitine, CoQ10, and N-acetylcysteine demonstrated statistically significant improvements in sperm motility compared with placebo (P < 0.05) (Supplemental Figure 5). The network meta-analysis (NMA) further confirmed that carnitine (mean difference [MD] = 12.43; 95% confidence interval [CI]: 4.07, 20.26) and CoQ10 (MD = 7.33; 95% CI: 0.35, 14.17) significantly enhanced sperm motility relative to placebo.
However, the NMA did not find statistically significant differences between placebo and other antioxidants including folic acid, zinc, vitamin E, selenium, and N-acetylcysteine. When comparing the relative rankings of effectiveness using surface under the cumulative ranking curve (SUCRA) scores, carnitine ranked highest (SUCRA = 88.7%), followed by selenium (71.1%) and CoQ10 (65.3%) (Supplemental Figure 6).
Sperm Morphology
In the pairwise meta-analysis, vitamin C, CoQ10, selenium, and N-acetylcysteine were associated with statistically significant improvements in sperm morphology compared with placebo (P < 0.05) (Supplemental Figure 7). However, when evaluated through NMA, no antioxidant demonstrated a statistically significant advantage over placebo for this outcome. The absence of statistical significance in the NMA suggests the need for cautious interpretation of the direct pairwise findings.
Despite the lack of definitive statistical superiority, the SUCRA rankings identified vitamin C as the top-ranking antioxidant for improving morphology (SUCRA = 93.6%), followed by CoQ10 (76.4%) and N-acetylcysteine (53.3%) (Supplemental Figure 8). These rankings provide a tentative hierarchy but should be interpreted conservatively due to overlapping confidence intervals and the nonsignificant NMA comparisons.
Inconsistency and Convergence Analysis
To assess the agreement between direct and indirect evidence, node-splitting analysis was conducted. No significant inconsistencies were found for any of the primary outcomes: sperm concentration, motility, or morphology. These findings support the validity of the consistency model used in the NMA. Furthermore, model convergence was confirmed using the potential scale reduction factor (PSRF), which approached 1 across all parameters, indicating strong convergence of the Bayesian model.
Sensitivity Analyses
Robustness of the findings was assessed via sensitivity analyses, which excluded studies with a high risk of bias (27, 30, 37) and those relying on imputed data (26, 32). The results remained consistent with those from the primary analysis, reaffirming the reliability of the findings.
For sperm concentration, CoQ10 retained the highest SUCRA (80.4%). For sperm motility, carnitine remained the top-ranking intervention (SUCRA = 86.2%), while vitamin C continued to rank highest for morphology (SUCRA = 89.3%). Additionally, node-splitting analyses performed during the sensitivity checks did not reveal any significant inconsistencies across all outcomes.
Assessment of Small Study Effects
To evaluate the potential for small study effects and publication bias, comparison-adjusted funnel plots were generated for each outcome. The visual inspection of the funnel plots revealed no substantial asymmetry for sperm concentration, motility, or morphology. This suggests a low likelihood that the overall results were substantially influenced by publication bias or selective reporting based on study size.
Discussion
This network meta-analysis (NMA), incorporating data from 18 randomized controlled trials (RCTs) involving 1790 subfertile men, evaluated the comparative efficacy of several antioxidant supplements in improving sperm quality parameters. Three principal findings emerged: (1) Coenzyme Q10 (CoQ10) appeared most effective in enhancing sperm concentration (SUCRA: 79.4%), (2) carnitine ranked highest for improving sperm motility (SUCRA: 88.7%), and (3) vitamin C was most favorable for sperm morphology (SUCRA: 93.6%). While these rankings offer insight into potential clinical applications, it is important to interpret them cautiously due to the absence of statistically significant differences between many of the interventions. Sensitivity analyses—excluding high-risk bias studies and those using imputed data—produced consistent results across all three parameters, reinforcing the robustness of our findings.
Prior meta-analyses have generally supported the beneficial impact of antioxidants on sperm function, with many suggesting improvements in parameters such as motility and morphology (14, 45). This has also been supported by in vitro studies demonstrating reduced oxidative damage following antioxidant exposure (46). However, previous meta-analyses largely treated antioxidant use as a homogenous intervention and did not differentiate between the individual agents. Thus, authors have called for NMAs to identify which specific antioxidants may yield the most significant clinical benefits (47). To our knowledge, this study represents the first NMA to quantitatively assess and compare both the direct and indirect effects of individual antioxidants on sperm parameters in subfertile men.
The rationale behind the use of antioxidants in treating male subfertility centers on mitigating oxidative stress, which plays a central role in sperm dysfunction. Reactive oxygen species (ROS) can impair sperm motility, DNA integrity, and membrane structure. CoQ10, a lipid-soluble antioxidant, is a key regulator of cellular energy metabolism and a stabilizer of lipid membranes. It inhibits lipid peroxidation in sperm plasma membranes, which are rich in polyunsaturated fatty acids and particularly vulnerable to oxidative insult (9). Additionally, CoQ10 modulates mitochondrial energy production, which is crucial for sperm motility. It is highly concentrated in the sperm midpiece, the site of mitochondrial energy generation, and lower CoQ10 levels have been reported in the semen of men with asthenozoospermia (49). Our findings not only align with these biological mechanisms but also show that CoQ10 significantly improved both sperm concentration and motility. Moreover, CoQ10 has been reported to act as a recycler of vitamin E and modulator of autophagy and membrane dynamics, further supporting its potential role in enhancing spermatogenesis (50).
Carnitine, a small water-soluble molecule, also demonstrates several antioxidant properties. The bioactive forms—L-carnitine and L-acetylcarnitine—neutralize superoxide anions and hydrogen peroxide, thereby mitigating oxidative damage and preventing lipid peroxidation. Carnitine also serves as a vital transporter of long-chain fatty acids into mitochondria, where they are oxidized for ATP generation (49). Its concentration in epididymal plasma and sperm cells is approximately 2000 times greater than in systemic circulation, highlighting its central role in sperm maturation (52). Given that fatty acid oxidation is the predominant energy source in spermatozoa during their maturation in the epididymis, carnitine’s energy-supplying role may explain its strong effect on motility observed in this NMA.
Vitamin C, a water-soluble antioxidant, operates via direct neutralization of several ROS species, including hydroxyl radicals, superoxide, and hydrogen peroxide (53). In seminal plasma, vitamin C is present at concentrations approximately 10 times higher than in serum (54), and higher seminal concentrations have been associated with improved sperm morphology and decreased DNA fragmentation (55). In our analysis, vitamin C emerged as the top-ranking antioxidant for improving sperm morphology, although the NMA did not show a statistically significant difference from other interventions or placebo. Nonetheless, the biological plausibility and strong SUCRA ranking make vitamin C a compelling candidate for future research, particularly given its wide availability, low cost, and favorable safety profile.
This study offers clinically relevant insight into tailoring antioxidant therapy based on specific semen parameter abnormalities. For instance, CoQ10 may be especially beneficial in cases of oligozoospermia (low sperm count), while carnitine may be more suitable for asthenozoospermia (reduced sperm motility). Given the relative safety and accessibility of vitamin C, it may be considered for improving morphology in men with teratozoospermia. These targeted recommendations provide a foundation for personalized antioxidant therapy in male infertility.
Importantly, the current clinical application of antioxidant therapy is hampered by the lack of standardized measures to assess redox status in patients. Although the ideal strategy would involve tailoring supplementation to an individual’s oxidative stress profile, no universally accepted method exists to define normal or pathological redox thresholds in semen (56). Therefore, clinical decisions often rely on empirical evidence and general semen analysis results. Our SUCRA-based rankings may offer a useful surrogate for selecting candidate antioxidants when personalized biochemical profiling is unavailable.
While this study adheres closely to the PRISMA extension guidelines for NMAs and provides the most comprehensive comparison of antioxidants to date, several limitations must be acknowledged. First, many of the comparisons between antioxidants are based on indirect evidence due to the limited number of head-to-head RCTs. This may restrict the strength and confidence in comparative conclusions. Second, the ultimate goal in fertility treatment—achieving clinical pregnancy or live birth—was not assessed in this study. Although improvements in semen parameters are suggestive of increased fertility potential, they do not guarantee improved reproductive outcomes, especially when female partner factors are not accounted for (10). Third, the current evidence base is insufficient to evaluate dose-response relationships. With more data, future NMAs could stratify outcomes by dosage and duration of antioxidant use to identify optimal regimens. Fourth, not all known antioxidants were included. For example, arginine was excluded due to the absence of eligible trials, and carotenoids were omitted because of their heterogeneous molecular forms (e.g., β-carotene, lycopene, lutein), which differ significantly in structure and biological activity.
Conclusion
This network meta-analysis of 18 randomized controlled trials involving 1790 subfertile men provides important insights into the comparative effectiveness of various antioxidants on sperm quality parameters. The findings indicate that Coenzyme Q10 (CoQ10) appears to be the most effective antioxidant for improving sperm concentration, likely due to its role in mitochondrial energy metabolism and antioxidative properties. Carnitine showed the greatest benefit in enhancing sperm motility, reflecting its function in fatty acid transport and protection against oxidative damage in sperm cells. Vitamin C ranked highest for improving sperm morphology, although the differences between antioxidants and placebo in morphology outcomes were not statistically significant. While these rankings offer useful guidance, the lack of direct head-to-head comparisons and limited reporting of reproductive outcomes such as pregnancy rates or live births temper the conclusions. Sensitivity analyses confirmed the robustness of these findings, but the evidence base remains constrained by a small number of trials and variability in study quality. Moreover, factors such as female partner fertility and other confounders were not consistently accounted for in the included studies. Despite these limitations, this study is the first to systematically compare multiple antioxidants using a network meta-analysis approach, providing a valuable reference for optimizing antioxidant therapy in male subfertility. Future research should focus on larger, well-designed trials incorporating clinical pregnancy outcomes and dose-response relationships to better define optimal antioxidant interventions and improve fertility outcomes.
Implications for Practice
Clinicians managing male subfertility often face uncertainty in selecting the most appropriate antioxidant therapy due to limited comparative evidence. This study offers practical guidance by suggesting that antioxidant supplementation can be tailored to specific sperm abnormalities identified in semen analysis. For men with oligozoospermia (low sperm concentration), CoQ10 supplementation may be most beneficial given its role in energy metabolism and antioxidant defense. For those with asthenozoospermia (poor sperm motility), carnitine appears to be the preferred antioxidant due to its involvement in fatty acid oxidation and protective effects against oxidative stress. Vitamin C may be the best option to improve sperm morphology, supported by its antioxidant capacity and high concentration in seminal plasma. Vitamin C is also a safe, cost-effective, and readily available supplement, making it a practical choice for routine dietary support. However, clinicians should be cautious, as improvements in sperm parameters do not guarantee increased pregnancy rates, and antioxidant therapy should be part of a holistic fertility management plan considering both partners. One key challenge is the current lack of standardized tests to measure oxidative stress or redox balance, limiting personalized antioxidant dosing. Developing such diagnostic tools would enhance treatment precision. Until more definitive clinical evidence and guidelines emerge, antioxidant therapy can be considered a complementary approach tailored to individual sperm abnormalities, potentially improving outcomes for subfertile men.
Acknowledgements
The authors thank all study participants and clinical staff involved in the original trials included in this meta-analysis. We also acknowledge the assistance of the University of Health Sciences Library team for their support with literature search strategies. This work was supported in part by a grant from the National Institute of Reproductive Health Research (Grant No. NIRHR-2023-045).
Conflict of Interest
The authors declare no conflicts of interest related to this study. None of the authors have received funding or fees from any commercial entities involved in antioxidant supplements or fertility treatments.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundl
- Definition and prevalence of subfertility and infertility. Hum Reprod 2005;20(5):1144–7.
- Del Giudice F, Busetto GM, De Berardinis E, Sperduti I, Ferro M, Maggi M, Gross MS, Sciarra A, Eisenberg A systematic review and meta- analysis of clinical trials implementing aromatase inhibitors to treat male infertility. Asian J Androl 2020;22(4):360–7.
- Daneshmandpour Y, Bahmanpour Z, Hamzeiy H, Mazaheri Moghaddam M, Mazaheri Moghaddam M, Khademi B, Sakhinia
- MicroRNAs association with azoospermia, oligospermia, asthenozoospermia, and teratozoospermia: a systematic review. J Assist Reprod Genet 2020;37(4):763–75.
- Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 2017;23(6):646–59.
- Colpi GM, Francavilla S, Haidl G, Link K, Behre HM, Goulis DG, Krausz C, Giwercman A. European Academy of Andrology guideline management of oligo-astheno-teratozoospermia. Andrology 2018;6(4):513–24.
- Oumaima A, Tesnim A, Zohra H, Amira S, Ines Z, Sana C, Intissar G, Lobna E, Ali J, Meriem M. Investigation on the origin of sperm morphological defects: oxidative attacks, chromatin immaturity, and DNA Environ Sci Pollut Res Int 2018;25(14):13775–86.
- Martins da Male infertility and antioxidants: one small step for man, no giant leap for andrology? Reprod Biomed Online 2019;39(6):879–83.
- Dias TR, Martin-Hidalgo D, Silva BM, Oliveira PF, Alves MG. Endogenous and exogenous antioxidants as a tool to ameliorate male infertility induced by reactive oxygen Antioxid Redox Signal 2020;33(11):767–85.
- Lafuente R, González-Comadrán M, Solà I, López G, Brassesco M, Carreras R, Checa MA. Coenzyme Q10 and male infertility: a meta- J Assist Reprod Genet 2013;30(9):1147–56.
- Agarwal A, Leisegang K, Majzoub A, Henkel R, Finelli R, Panner Selvam MK, Tadros N, Parekh N, Ko EY, Cho CL, et Utility of antioxidants in the treatment of male infertility: clinical guidelines based on a systematic review and analysis of evidence. The World Journal of Men’s Health 2021;39(2):233.
- Ross C, Morriss A, Khairy M, Khalaf Y, Braude P, Coomarasamy A, El- Toukhy A systematic review of the effect of oral antioxidants on male infertility. Reprod Biomed Online 2010;20(6):711–23.
- Busetto GM, Agarwal A, Virmani A, Antonini G, Ragonesi G, Del Giudice F, Micic S, Gentile V, De Berardinis Effect of metabolic and antioxidant supplementation on sperm parameters in oligo-astheno- teratozoospermia, with and without varicocele: a double-blind placebo- controlled study. Andrologia 2018;50(3): e12927.
- Comhaire F, Decleer W. Quantifying the effectiveness and cost- efficiency of food supplementation with antioxidants for male Reprod Biomed Online 2011;23(3):361–2.
- Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, Showell Antioxidants for male subfertility. Cochrane Database Syst Rev 2019;3(3):Cd007411.
- Schwingshackl L, Schwarzer G, Rücker G, Meerpohl JJ. Perspective: network meta-analysis reaches nutrition research: current status, scientific concepts, and future directions. Adv Nutr 2019;10(5): 739–54.
- Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and Ann Intern Med 2015;162(11): 777–84.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343(oct18 2):d5928.
- Schwingshackl L, Neuenschwander M, Hoffmann G, Buyken AE, Schlesinger S. Dietary sugars and cardiometabolic risk factors: a network meta-analysis on isocaloric substitution interventions. Am J Clin Nutr 2020;111(1):187–96.
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8(10):e76654.
- Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care 1990;6(1):5–30.
- Higgins JPT, Green Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011.
- Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27(6):1785–805.
- Wan X, Wang W, Liu J, Tong Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Method 2014;14(1):135.
- Jansen JP, Crawford B, Bergman G, Stam Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value in Health 2008;11(5):956–64.
- Dias S, Welton NJ, Caldwell DM, Ades Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29(7– 8):932–44.
- Wong WY, Merkus H, Thomas CMG, Menkveld R, Zielhuis GA, Steegers-Theunissen Effects of folic acid and zinc sulfate on male factor subfertility: a double-blind, randomized, placebo-controlled trial. Fertil Steril 2002;77(3):491–8.
- Suleiman SA, Ali ME, Zaki ZM, el-Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl 1996;17(5):530–7.
- Sigman M, Glass S, Campagnone J, Pryor Carnitine for the treatment of idiopathic asthenospermia: a randomized, double-blind, placebo- controlled trial. Fertil Steril 2006;85(5):1409–14.
- Scott R, MacPherson A, Yates RW, Hussain B, Dixon J. The effect of oral selenium supplementation on human sperm motility. BJU Int 1998;82(1):76–80.
- Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: a double-blind, placebo controlled, randomized study. J Urol 2012;188(2):526–31.
- Safarinejad MR, Safarinejad S. Efficacy of selenium and/or N-acetyl- cysteine for improving semen parameters in infertile men: a double- blind, placebo controlled, randomized J Urol 2009;181(2):741– 51.
- Raigani M, Yaghmaei B, Amirjannti N, Lakpour N, Akhondi MM, Zeraati H, Hajihosseinal M, Sadeghi MR. The micronutrient supplements, zinc sulphate and folic acid, did not ameliorate sperm functional parameters in oligoasthenoteratozoospermic Andrologia 2014;46(9):956–62.
- Nadjarzadeh A, Sadeghi MR, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, Akhondi MA, Yavari P, Shidfar F. Coenzyme Q10 improves seminal oxidative defense but does not affect on semen parameters in idiopathic oligoasthenoteratozoospermia: a randomized double-blind, placebo controlled trial. J Endocrinol Invest 2011;34(8):e224–8.
- Mehni NM, Ketabchi AA, Hosseini Combination effect of pentoxifylline and L-carnitine on idiopathic oligoasthenoteratozoospermia. Iranian J Reprod Med 2014;12(12):817– 24.
- Lenzi A, Sgrò P, Salacone P, Paoli D, Gilio B, Lombardo F, Santulli M, Agarwal A, Gandini A placebo-controlled double-blind randomized trial of the use of combined L-carnitine and L-acetyl-carnitine treatment in men with asthenozoospermia. Fertil Steril 2004;81(6):1578–84.
- Eslamian G, Amirjannati N, Noori N, Sadeghi MR, Effects of coadministration of DHA and vitamin E on spermatogram, seminal oxidative stress, and sperm phospholipids in asthenozoospermic men: a randomized controlled trial. Am J Clin Nutr 2020;112(3):707–19.
- da Silva TM, Maia MCS, Arruda JT, Approbato FC, Mendonça CR, Approbato MS. Folic acid does not improve semen parametrs in subfertile men: a double-blin, randomized, placebo-controlled study. Jornal Brasileiro de Reproducao Assistida 2013;17(3):152–7.
- Cyrus A, Kabir A, Goodarzi D, Moghimi M. The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: a double blind placebo controlled clinical trial. Int Braz J Urol 2015;41(2):230–8.
- Cheng JB, Zhu J, Ni F, Jiang H. L-carnitine combined with coenzyme Q10 for idiopathic oligoasthenozoospermia: a double- blind randomized controlled trial. Zhonghua nan ke xue [National Journal of Andrology] 2018;24(1):33–8.
- Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro
- Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L- acetylcarnitine in men with idiopathic asthenozoospermia. Fertil Steril 2005;84(3):662–71.
- Balercia G, Buldreghini E, Vignini A, Tiano L, Paggi F, Amoroso S, Ricciardo-Lamonica G, Boscaro M, Lenzi A, Littarru G. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertil Steril 2009;91(5):1785–92.
- Alahmar AT, Sengupta P. Impact of coenzyme Q10 and selenium on seminal fluid parameters and antioxidant status in men with idiopathic Biol Trace Elem Res 2021; 199(4):1246–52.
- Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol 2009;182(1):237–48.
- Banholzer ML, Buergin H, Wandel C, Schmitt G, Gocke E, Peck R, Singer T, Reynolds T, Mannino M, Deutsch J, et al. Clinical trial considerations on male contraception and collection of pregnancy information from female partners. J Transl Med 2012;10(1):129.
- Salas-Huetos A, Rosique-Esteban N, Becerra-Tomás N, Vizmanos B, Bulló M, Salas-Salvadó J. The effect of nutrients and dietary supplements on sperm quality parameters: a systematic review and meta-analysis of randomized clinical trials. Adv Nutr 2018;9(6): 833–48.
- Iovine C, Mottola F, Santonastaso M, Finelli R, Agarwal A, Rocco In vitro ameliorative effects of ellagic acid on vitality, motility and DNA quality in human spermatozoa. Mol Reprod Dev 2021;88(2): 167–74.
- Bahmyari R, Zare M, Sharma R, Agarwal A, Halvaei The efficacy of antioxidants in sperm parameters and production of reactive oxygen species levels during the freeze-thaw process: a systematic review and meta-analysis. Andrologia 2020;52(3):e13514.
- Banihani SA. Effect of coenzyme Q(10) supplementation on Biomolecules 2018;8(4):172.
- Ihsan AU, Khan FU, Khongorzul P, Ahmad KA, Naveed M, Yasmeen S, Cao Y, Taleb A, Maiti R, Akhter F, et Role of oxidative stress in pathology of chronic prostatitis/chronic pelvic pain syndrome and male infertility and antioxidants function in ameliorating oxidative stress. Biomed Pharmacother 2018;106:714–23.
- Suárez-Rivero JM, Pastor-Maldonado CJ, Povea-Cabello S, Álvarez- Córdoba M, Villalón-García I, Munuera-Cabeza M, Suárez-Carrillo A, Talaverón-Rey M, Sánchez-Alcázar JA. Coenzyme Q(10) analogues: benefits and challenges for therapeutics. Antioxidants (Basel, Switzerland) 2021;10(2):236.
- Agarwal A, Said TM. Carnitines and male infertility. Reprod Biomed Online 2004;8(4):376–84.
- Ng CM, Blackman MR, Wang C, Swerdloff RS. The role of carnitine in the male reproductive system. Ann N Y Acad Sci 2004;1033(1): 177–88.
- Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci 1991;88(24):11003–6.
- Jacob RA, Pianalto FS, Agee Cellular ascorbate depletion in healthy men. J Nutr 1992;122(5):1111–8.
- Calogero AE, Condorelli RA, Russo GI, La Vignera S. Conservative nonhormonal options for the treatment of male infertility: antibiotics, anti-inflammatory drugs, and antioxidants. Biomed Res Int 2017;2017:1.
- Cardoso JP, Cocuzza M, Elterman D. Optimizing male fertility: oxidative stress and the use of antioxidants. World J Urol 2019;37(6):1029–34.

