Abstract:
Male-related factors account for approximately 20–30% of all infertility cases. Oxidative stress-induced DNA damage in sperm has been closely associated with oligoasthenoteratozoospermia (OAT) and subfertility in men. Antioxidant therapy is being investigated globally for its potential to alleviate OAT, minimize sperm DNA fragmentation, and counteract reactive oxygen species. This study aimed to evaluate the impact of an antioxidant formulation on sperm count, semen profile, and DNA fragmentation index (DFI) in men with subfertility. A double-blind, randomized, placebo-controlled, prospective clinical trial was undertaken, involving 300 male participants (aged 25–45 years) across ten clinical centers in India. Participants were randomly allocated to receive either the antioxidant combination or a placebo. Semen analysis included sperm concentration, motility, morphology, volume, and DFI, measured at baseline and following a 90-day intervention. Further subgroup analyses were conducted based on specific stratification criteria. Statistical processing utilized SPSS version 10.0. The treatment group exhibited significant enhancements in total sperm count, ejaculate volume, motility, and normal sperm morphology. Notably, individuals with severe oligospermia (sperm counts below 5 million/mL, between 5–10 million/mL, and 10.1–15 million/mL) and those with elevated baseline DFI levels (20–30%, 31–40%, and >40%) demonstrated marked improvements, as revealed in post hoc evaluations. No participants withdrew prematurely, and no adverse effects were observed, underscoring the safety and good tolerability of the antioxidant therapy. These findings reinforce the well-established role of antioxidants in mitigating oxidative damage, thereby preserving sperm DNA integrity and enhancing semen parameters, particularly among men over the age of 40.
Trial Registration: Clinical Trials Registry – India (CTRI) Identifier: CTRI/2020/12/029590
Keywords: Male Subfertility; Antioxidant Therapy; Asthenoteratozoospermia; Sperm DNA Integrity; Oligospermia
Introduction
Infertility remains a significant global health issue, with an estimated 60–80 million couples affected annually—representing approximately 8–10% of all couples [1]. According to the World Health Organization (WHO), infertility impacts one in four couples in developing nations. In India alone, an estimated 15–20 million couples (25%) face infertility each year [2], highlighting the pressing need for effective interventions. Male factors contribute solely to approximately 20–30% of infertility cases [3]. Semen abnormalities—such as oligospermia, asthenozoospermia, and teratozoospermia—are among the most commonly identified causes of male infertility [4]. The etiological factors of male infertility can be classified into three categories: pre-testicular causes include gonadotropin deficiencies, pituitary dysfunction, hyperprolactinemia, excess glucocorticoids, and thyroid imbalances; testicular causes encompass conditions like Klinefelter syndrome, undescended testes, trauma, varicocele, and testicular malignancies; and post-testicular causes involve congenital absence of the vas deferens, infections, surgical injury, sperm motility disorders, or ejaculatory/erectile dysfunctions. In nearly 30% of men diagnosed with infertility, no identifiable cause is found using standard diagnostic methods—this is termed idiopathic infertility [5]. Oligospermia, defined by WHO as sperm concentration below 15 million/mL [6]; asthenozoospermia, as motility less than 40% or progressive motility below 32% [7]; and teratozoospermia, which includes abnormal sperm morphology affecting various sperm structures—are commonly observed anomalies. When these defects co-occur, the condition is referred to as oligoasthenoteratozoospermia (OAT), one of the most prevalent clinical profiles of male infertility [8].
Various therapeutic strategies are currently in use, including surgical correction, hormone or drug therapy, intrauterine insemination (IUI), assisted reproductive technologies (ART), in vitro fertilization (IVF), and intracytoplasmic sperm injection (ICSI) [9]. However, these options often involve high costs, potential side effects, and limited success rates. Therefore, more affordable, well-tolerated, and clinically efficient alternatives are needed. Oxidative stress-induced sperm DNA damage is closely linked to OAT and impaired fertility outcomes [10]. Such DNA damage correlates with diminished reproductive potential, increased miscarriage rates, and poorer embryo development [11]. The DNA fragmentation index (DFI) quantifies the proportion of sperm with compromised DNA in a semen sample [9,12]. Owing to their accessibility, safety profile, and cost-effectiveness, antioxidants have attracted considerable attention for managing male subfertility. Commonly utilized compounds include vitamins C and E, carnitine, selenium, zinc, and N-acetyl cysteine [9,12]. Clinically, antioxidants have been used to improve semen parameters and DFI in men with oligospermia, thereby potentially enhancing fertility outcomes. Nonetheless, robust, controlled clinical data are essential to substantiate these effects [12]. This study aims to provide scientific evidence on the effectiveness of a specific antioxidant formulation in improving semen parameters and reducing DNA fragmentation in men diagnosed with oligospermia.
Methods
A prospective, randomized controlled clinical trial was carried out to evaluate the effects of an antioxidant supplement in men diagnosed with oligospermia. Recruitment was conducted across ten clinical centers in India, with site details available on the Clinical Trials Registry of India (CTRI) under the registration number CTRI/2020/12/029590. The study protocol received approval from the Royal Pune Independent Ethics Committee in Pune, Maharashtra. The trial’s design and subject allocation are illustrated in the Consolidated Standards of Reporting Trials (CONSORT) diagram (Fig. 1).
Inclusion Criteria
Participants included male subjects aged 25 to 45 years, presenting with oligospermia, asthenozoospermia, or teratozoospermia. All enrolled individuals provided written informed consent and agreed to complete study follow-ups. Female partners were confirmed to have no reported infertility issues. Based on WHO standards for oligoasthenoteratozoospermia (OAT), eligible participants met at least two of the following semen analysis criteria: semen volume under 1.5 mL, sperm count below 15 × 10⁶/mL, total motility under 25%, normal morphology under 4%, or DNA fragmentation index (DFI) greater than 20%.
Exclusion Criteria
Exclusion was applied to participants with known histories of hypogonadism, prior vasectomy, undescended testes, varicocele, hydrocele, prostate malignancies, or prior exposure to chemotherapy or radiation therapy. Individuals diagnosed with azoospermia were also excluded from the study.
Study Groups and Randomization
A total of 300 participants who met the eligibility criteria were randomized into two groups—treatment and placebo—using a computer-generated randomization schedule. The treatment arm received an antioxidant combination in tablet form, while the control arm received a placebo.
Sample Size Justification
Assuming a 25% difference in sperm count between the treatment and placebo groups, a total sample size of 300 (150 per group) was calculated to provide 90% statistical power at a 5% significance level. The calculation was performed using the online tool available at https://clincalc.com/stats/samplesize.aspx.
Details of the Intervention
The treatment group received a daily antioxidant formulation containing a comprehensive mix of vitamins, amino acids, trace elements, and plant extracts with known fertility-enhancing properties. The active constituents per tablet are outlined in Table 1.
Therapeutic Justification
Male infertility remains a complex clinical issue, particularly in cases involving OAT where combined qualitative and quantitative sperm impairments are common. Literature reviews, including those indexed on PubMed, Google Scholar, Embase, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews—as per the PRISMA guidelines—support the therapeutic relevance of dietary and antioxidant interventions in managing male infertility [11]. Numerous clinical investigations support the use of antioxidants for treating idiopathic infertility. Their global application in OAT cases is grounded in their established safety and observed improvements in sperm function.
| Ingredients | Label Claim (per tablet) |
| Coenzyme Q10 | 50 mg |
| L-carnitine | 50 mg |
| Vitamin C | 40 mg |
| Vitamin E | 10 mg |
| Ginseng extract | 10 mg |
| L-arginine | 10 mg |
| Zinc (as zinc sulfate) | 7.5 mg (20.588 mg) |
| Iron (as ferrous fumarate) | 5 mg (15.21 mg) |
| L-glutathione | 2.5 mg |
| Vitamin B6 | 2 mg |
| Manganese (as manganese sulfate) | 2 mg (6.152 mg) |
| Lycopene | 2 mg |
| Vitamin B1 | 1.4 mg |
| Copper (as cupric sulfate) | 1 mg (2.795 mg) |
| Vitamin A | 375 mcg (1,250 IU) |
| Folic acid | 100 mcg |
| Selenium (as sodium selenate) | 40 mcg (95.716 mcg) |
| Vitamin D | 10 mcg (400 IU) |
| Vitamin B12 | 1 mcg |
Rationale Behind Antioxidant Use
Under physiological conditions, reactive oxygen species (ROS) play essential roles in sperm development and fertilization, including capacitation, hyperactivation, and the acrosome reaction. However, when ROS levels become excessively elevated—due to internal or environmental influences—they can trigger lipid peroxidation, DNA fragmentation, and apoptosis in sperm cells, ultimately impairing fertility [11,13]. Antioxidant supplementation offers a potential therapeutic pathway for mitigating ROS-induced sperm DNA damage. Given the increasing clinical interest in non-invasive, cost-effective fertility therapies, this study was designed to evaluate the therapeutic potential of an antioxidant formulation in improving semen quality and DNA integrity among sub-fertile men.
Dosage and Administration
Participants assigned to either the treatment or placebo group were instructed to take one tablet per day, orally, with water following their main meal for a total duration of 90 days from the start of the study. The antioxidant and placebo tablets were designed to appear identical to maintain the double-blind nature of the trial. The dosage composition adhered to regulatory standards and fell within the Recommended Dietary Allowance (RDA) limits for all included ingredients (see Table 1).
Outcome Measures
The primary endpoints of this clinical trial were the assessment of changes in total sperm count and DNA fragmentation index (DFI) after 90 days of intervention in sub-fertile males. The secondary outcomes included evaluations of alterations in semen volume, sperm motility, and sperm morphology over the same period.
Study Schedule
This study was a prospective, randomized, double-blind, placebo-controlled, and multicentric trial, carried out in 300 sub-fertile male participants at ten clinical centers across India. At the initial (screening) visit, informed consent was obtained from all potential subjects, followed by documentation of demographic data. Subjects underwent clinical evaluations at each visit, and semen samples were analyzed both at baseline and on day 90. Parameters assessed included sperm count, motility, morphology, semen volume, color, pH, liquefaction, and DFI. DNA fragmentation testing was conducted at The Andrology Center in Coimbatore, Tamil Nadu (PIN 641018), using the Sperm Chromatin Structure Assay. Participants were instructed to maintain a three-day period of sexual abstinence before sample collection. Subjects were randomized into either the antioxidant treatment or placebo group using a computer-generated allocation sequence in a 1:1 ratio. Study medications were dispensed in opaque, high-density polyethylene containers, each holding 35 identically appearing tablets to preserve blinding. Tablets were administered once daily after the main meal, as per protocol. Subjects were instructed not to take any other antioxidant formulations, multivitamins, nutraceuticals, herbal supplements, or traditional medicines during the study period. Monthly follow-ups were scheduled to monitor progress and assess compliance. Pregnancy outcomes were recorded, and participants continued with the treatment regimen even if conception occurred, in order to complete the full study duration. Compliance was evaluated during each follow-up by counting the returned unused tablets. Subjects who missed more than three consecutive doses or exceeded six missed doses within any 30-day period were considered dropouts.
Statistical Analysis
All data analyses were conducted on a per-protocol population. Statistical evaluations were carried out using SPSS software version 10.0 (SPSS Inc., Chicago, IL, USA). For normally distributed variables, data were summarized as mean ± standard deviation, while non-normally distributed variables were presented as median with interquartile ranges. The Shapiro-Wilk test was applied to assess the normality of distributions. Intergroup comparisons were made using either the independent samples t-test or the Mann-Whitney U test for non-parametric data. A p-value ≤ 0.05 was regarded as statistically significant throughout the study.
Results
The mean age of participants was statistically comparable between the two groups: 35 ± 5.1 years in the treatment group and 34 ± 5.0 years in the placebo group.
Change in Total Sperm Count in Sub-fertile Males
At baseline, the average sperm count was similar in both groups: 27.56 ± 20.69 million/mL in the treatment group and 27.15 ± 18.55 million/mL in the placebo group. Following 90 days of intervention, a 42.41% increase was observed in the treatment group (mean: 39.25 ± 80.32 million/mL), compared to an 11.04% increase in the placebo group (mean: 30.15 ± 19.38 million/mL). Intragroup analysis showed statistically significant improvements from baseline in both groups (dependent Student’s t-test), although the between-group difference in sperm count at the end of the study did not reach statistical significance (Table 2).
A post hoc subgroup analysis based on initial sperm counts revealed more pronounced effects of the antioxidant treatment. Among subjects with baseline sperm counts below 5 million/mL, the treatment group showed a 76.75% improvement, while the placebo group exhibited only a 5.57% rise. In the 5–10 million/mL and 10–15 million/mL subgroups, the treatment group demonstrated increases of 73.62% and 43.72%, respectively—both substantially greater than those observed in the placebo group (Figure 2).
Further stratification by age showed that in subjects over 40 years, the antioxidant group achieved a 31.33% improvement in sperm count compared to 4.52% in the placebo group (n = 25 and n = 20, respectively), which was statistically significant (Table 2).
Change in Sperm Motility
Sperm motility improved significantly in both groups. The antioxidant group experienced a 27.35% rise (from 17.18% to 21.88%; p = 0.0010), while the placebo group showed a 28.41% improvement (from 17.84% to 23.03%; p = 0.0043). Despite the within-group gains, no significant difference was observed between groups at the end of the study (Table 2).
Among participants aged over 40, sperm motility increased by 42.01% in the antioxidant group—a statistically significant change. The placebo group showed a non-significant 20.87% increase (Table 2).
Table 2. Mean changes in semen parameters after 90 days of intervention
| Parameter | Baseline | p-value (between groups) | Day 90 (p-value within group) | p-value (between groups) |
| Average sperm count (million/mL) | Treatment: 27.56 ± 20.69Placebo: 27.15 ± 18.55 | 0.8690 | Treatment: 39.25 ± 80.32 (p = 0.0800)Placebo: 30.15 ± 19.38 (p = 0.7700) | 0.1639 |
| Average sperm motility (%) | Treatment: 17.18 ± 11.95Placebo: 17.84 ± 11.70 | 0.6603 | Treatment: 21.88 ± 14.21 (p = 0.0010)Placebo: 23.03 ± 22.09 (p = 0.0043) | 0.7878 |
| Average DFI (%) | Treatment: 32.59 ± 12.67Placebo: 31.38 ± 10.71 | 0.8219 | Treatment: 26.81 ± 11.61 (p = 0.0073)Placebo: 28.45 ± 14.35 (p = 0.0688) | 0.6218 |
| Average semen volume (mL) | Treatment: 1.77 ± 0.92Placebo: 1.82 ± 0.91 | 0.6805 | Treatment: 2.00 ± 0.89 (p = 0.0032)Placebo: 2.00 ± 1.90 (p = 0.2412) | 0.8617 |
| Average sperm morphology (%) | Treatment: 3.46 ± 1.84Placebo: 4.07 ± 3.46 | 0.0873 | Treatment: 4.28 ± 1.91 (p = 0.0010)Placebo: 4.31 ± 2.28 (p = 0.2859) | 0.0199 |
Values are shown as mean ± standard deviation. Statistical comparisons used Student’s t-test. Significance was set at p < 0.05. Sample sizes: semen analysis (n = 125 treatment, n = 128 placebo), DFI analysis (n = 66 treatment, n = 75 placebo), age ≥ 40 group (n = 25 treatment, n = 20 placebo).
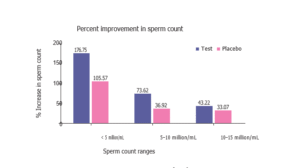
Figure 2. Percentage increase in sperm count across baseline ranges
Change in Sperm Motility in Sub-fertile Male Subjects
A significant improvement in sperm motility was observed in both groups. In the antioxidant-treated group, motility increased by 27.35%, from 17.18% to 21.88% (p = 0.0010), while the placebo group showed a comparable 28.41% rise, from 17.84% to 23.03% (p = 0.0043). Among participants aged 40 years and above, the antioxidant group experienced a statistically significant 42.01% increase in motility, whereas the placebo group had a non-significant increase of 20.87% (Table 2).
Change in DNA Fragmentation Index (DFI)
In subjects with a baseline DFI > 20%, treatment with the antioxidant blend significantly reduced the mean DFI from 32.59 ± 12.67% to 26.81 ± 11.61% (p = 0.0073). Notably, in the subgroup aged 40 years and older, the DFI reduction was greater in the treatment group (15.74%) compared to the placebo group (4.97%) (Table 2). Further stratification based on baseline DFI levels (20–30%, 31–40%, and >40%) provided additional insights (Table 3). In the 31–40% group, the antioxidant treatment yielded a statistically significant reduction in DFI (8.31 ± 15.05%, p = 0.0431), comparable to the placebo group (9.44 ± 8.34%, p = 0.0093). In the >40% subgroup, the antioxidant group showed a more pronounced DFI reduction (22.93 ± 19.55%, p = 0.0004) compared to the placebo group (13.17 ± 19.39%, p = 0.0383). No significant differences were observed in the 20–30% DFI subgroup.
Table 3. Reduction in DNA Fragmentation Index (DFI) by baseline DFI strata
| DFI Range | Timepoint | Treatment (n = 42) | Placebo (n = 43) | p-value |
| 20–30% | Screening | 24.03 ± 2.56 | 25.05 ± 2.87 | 0.1057 |
| Day 90 | 26.08 ± 10.97 | 27.03 ± 10.90 | — | |
| Mean difference | −2.05 ± 11.60 | −1.97 ± 11.59 | 0.9763 | |
| p-value | 0.2824 | 0.3073 | — | |
| 31–40% | Screening | 35.56 ± 2.83 | 33.89 ± 2.03 | 0.1328 |
| Day 90 | 27.25 ± 13.23 | 24.44 ± 8.55 | — | |
| Mean difference | 8.31 ± 15.05 | 9.44 ± 8.34 | 0.8376 | |
| p-value | 0.0431 | 0.0093 | — | |
| >40% | Screening | 51.13 ± 13.26 | 49.00 ± 9.25 | 0.6409 |
| Day 90 | 28.20 ± 12.06 | 35.83 ± 23.36 | — | |
| Mean difference | 22.93 ± 19.55 | 13.17 ± 19.39 | 0.2073 | |
| p-value | 0.0004 | 0.0383 | — |
Values are expressed as mean ± standard deviation. Data were analyzed using Student’s t-test. Statistical significance was considered at p < 0.05.
Change in Semen Volume
Mean semen volume significantly increased in the antioxidant group by 12.99%, from 1.77 mL to 2.00 mL (p = 0.0032). The placebo group also showed a statistically significant increase of 9.89% (from 1.82 mL to 2.00 mL), though the change was not as pronounced. In participants over 40, the antioxidant group experienced a 12.65% increase, whereas the placebo group showed a slight decrease in volume (Table 2).
Change in Sperm Morphology
Normal sperm morphology improved in both groups. The antioxidant-treated group demonstrated a significant increase from 3.46% to 4.28% (p = 0.0010), while the placebo group saw a smaller, non-significant change from 4.07% to 4.31%. Among individuals over 40 years of age, improvement in sperm morphology was also evident in both groups, although statistically significant only in the placebo group (Table 2).
Discussion
There were improvements in sperm count, semen volume, sperm motility, and sperm normal morphology in the treatment group. The subject population included men aged 40 years and above, a group in which antioxidant blend treatment significantly improved semen parameters compared to placebo. Notably, improvements were observed in sperm count among men with severe oligospermia (sperm counts < 5 million/mL, 5–10 million/mL, and 10.1–15 million/mL), as well as in men with high to extremely high baseline DNA fragmentation index (DFI) levels (20–30%, 31–40%, and above 40%) when the data was stratified post hoc. Importantly, there were no premature discontinuations or adverse events reported during the study, indicating the treatment is safe and well tolerated. The interventional antioxidant blend tablet is a combination of micronutrients, essential amino acids, antioxidants, and vitamins that are critical for the male reproductive system. These nutrients support various physiological processes important for sperm production, maturation, and DNA integrity. Clinical experience and literature reports suggest that, after adjusting for female age, conception during a 12-month period is approximately 30% less likely for men over 40 years of age compared to men younger than 30 years [14]. Advanced paternal age has a significant detrimental effect on sperm chromatin integrity, with a higher prevalence of elevated DFI (>10%) in men aged 40 and above. Moreover, both sexual function and semen parameters tend to decline with age after 40 years in males [14]. In our study, post hoc stratification by age showed a clear improvement in semen parameters among men aged 40 years and older following antioxidant treatment.
Numerous studies worldwide have confirmed the important role of nutrients, vitamins, and minerals in supporting sperm health [15]. Sperm DNA fragmentation is clinically correlated with infertility and is associated with increased rates of spontaneous abortion and diminished embryo quality. When DFI exceeds 25%, current literature suggests therapeutic interventions such as antioxidants, medical treatment, and lifestyle changes should be employed [16]. The antioxidant blend used in this study contains vitamins A, D, E, C, B12, and B6, among others. Clinical trials worldwide have demonstrated the potential of these vitamins in reducing sperm DNA fragmentation [17]. Some studies have shown that a combination of vitamins with micronutrients like zinc, selenium, manganese, copper, and iron can reduce DFI by approximately 15–17% [18]. Antioxidants in the form of essential amino acids, vitamins, and minerals are vital for energy metabolism and spermatozoa maturation, helping maintain sperm DNA integrity. Additionally, the inclusion of ginseng in some formulations has been demonstrated to reduce DFI in sub-fertile males [14,19].
Our study results confirmed that the antioxidant blend demonstrated clinical superiority over placebo by significantly reducing DFI in subjects with baseline DFI above 30–40%, as well as producing around a 15% reduction in DFI for men aged 40 years and older. This finding has important clinical implications, suggesting that this antioxidant blend could be an effective treatment option for sub-fertile men aged 40 and above. There is abundant published research on the benefits of antioxidant treatment, especially for idiopathic or unexplained male infertility [20–23]. Vitamin C, for instance, is well recognized for protecting sperm from oxidative damage. Supplementation with vitamin C has been shown to improve sperm quality in smokers by reducing sperm agglutination, ultimately increasing fertility [23].
Zinc deficiency has been associated with reduced sperm count and impotence in men. Supplementation with zinc improves sperm count and overall reproductive health [24]. Selenium supplementation has also been shown to improve sperm motility. L-arginine, an essential amino acid, is required for sperm production. Studies globally have found that nutrients such as vitamin A, vitamin E, zinc, and selenium are favorably related to androgen deficiency and sperm production [25]. Vitamin B12 supplementation has similarly been demonstrated to increase sperm counts. Coenzyme Q10, an antioxidant, improves sperm function and is useful in treating asthenozoospermia [26]. Vitamin E supplementation may enhance fertility by reducing free-radical damage to sperm cells; it supports spermatogenesis, increases androgen secretion, reduces oxidative stress, nourishes sperm, and promotes overall well-being [27].
According to current literature, oxidative stress is responsible for 30% to 80% of male sub-fertility cases, damaging spermatozoa and decreasing the success rate of assisted reproductive technologies (ART). Oral antioxidant interventions such as myo-inositol, alpha-lipoic acid, folic acid, coenzyme Q10, zinc, selenium, and various vitamins have shown significant improvements in semen reproductive potential and clinical outcomes of intracytoplasmic sperm injection (ICSI) [28]. Past research has also observed that antioxidant supplementation improves sperm competence, as evidenced by significantly better embryo quality during ART cycles following treatment, including a higher proportion of embryos with good morphology compared to baseline cycles [28]. A Cochrane Database Systematic Review by Showell et al. [29], which included randomized controlled trials involving 2,876 couples using various antioxidant compounds, showed a positive impact on live birth and pregnancy rates among sub-fertile couples undergoing ART cycles. While some studies reported on pregnancy outcomes, others focused primarily on sperm parameters. Our present study aligns with these findings, providing scientific evidence that antioxidant treatment can improve DFI and other semen parameters [29]. This reinforces the value of antioxidant therapy as a treatment strategy for male infertility and suggests its potential to improve prognosis for intrauterine insemination (IUI), ART, or in vitro fertilization (IVF) outcomes.
Importantly, our study is the first to provide evidence for the safety of this antioxidant blend tablet administered for three months, with no adverse effects observed in any participants. A key strength of this study is that semen samples for DFI analysis were processed at a centralized laboratory, minimizing bias and subjectivity, thereby supporting the reproducibility and robustness of our findings. We acknowledge certain limitations, including the use of male age as a confounder without controlling for nutritional status, body mass index, or partner age. Additionally, pregnancy outcomes were not assessed post-treatment; thus, future clinical trials of longer duration are warranted to evaluate these endpoints. In summary, this study demonstrates significant improvements in sperm count, semen volume, DFI, sperm motility, and sperm normal morphology in men receiving the antioxidant blend. Our results support the well-researched premise that antioxidants reduce oxidative stress and improve sperm DNA integrity. Notably, the antioxidant blend was effective in improving semen parameters in men aged 40 years and above. No adverse events were reported during the study, indicating that the antioxidant blend tablet is safe and well tolerated. Oligospermia remains the most common cause of male infertility. Although many nutraceuticals have traditional claims of fertility enhancement, this antioxidant blend tablet has shown promising effects in clinical trials. We propose that this antioxidant blend could improve both the quality and quantity of sperm in sub-fertile males, including aging men aged 40 years and above.
Conclusion
In conclusion, this study demonstrated that supplementation with the antioxidant blend tablet significantly improved key semen parameters including sperm count, semen volume, sperm motility, and normal sperm morphology in sub-fertile men. Notably, the treatment was especially effective in men aged 40 years and above, a population known to experience age-related declines in fertility and increased sperm DNA fragmentation. The antioxidant blend also produced a significant reduction in DNA fragmentation index (DFI), particularly in men with high baseline DFI values, which is strongly associated with poor fertility outcomes. These improvements suggest that the antioxidant formulation can mitigate oxidative stress, a major contributor to sperm damage and male infertility. Importantly, the treatment was well tolerated with no adverse events reported, confirming its safety for use over a three-month period. The study’s rigorous methodology, including centralized DFI testing, enhances the reliability of the results. While male age was considered as a confounder, future studies should incorporate additional factors such as nutritional status and partner age, and assess pregnancy outcomes over longer periods. Overall, the antioxidant blend tablet represents a promising, non-invasive intervention to improve semen quality and DNA integrity in sub-fertile men, particularly those of advanced paternal age. Its use may contribute to improved fertility and better reproductive success in assisted reproductive technologies.
Acknowledgments
The authors wish to thank all the study participants for their valuable time and cooperation. We acknowledge the support of the clinical staff at all participating centers. We also thank the laboratory team at The Andrology Center, Coimbatore for conducting the DFI assays. This study was funded by MedLife Pharmaceuticals Pvt. Ltd.
Conflict of Interest
The authors declare no conflict of interest related to this study. MedLife Pharmaceuticals provided funding and the antioxidant blend tablets but had no role in data analysis or manuscript preparation.
REFERENCES
- Katole A, Saoji AV. Prevalence of primary infertility and its associated risk factors in urban population of central India: a community-based cross-sectional study. Indian J Community Med 2019;44:337-341.
- World Health Organization. Infertility: a tabulation of available data on prevalence of primary and secondary infertility. Geneva: World Health Organization; 1991.
- Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015;13:37.
- Karavolos S, Stewart J, Evbuomwan I, McEleny K, Aird I. Assessment of the infertile male. Obstet Gynecol 2013;15:1-9.
- Hossain MA, Naser MF, Azam MS, Islam MW, Biswas NP, Bhuiyan AM, et al.. Male Infertility: Causes and Optimal Evaluation. Bangladesh J Urol 2016;9:43-8.
- World Health Organization. WHO laboratory manual for the examination and processing of human semen. Geneva: World Health Organization; 2010.
- Cavallini G. Clinical management of male infertility. Cham: Springer; 2015.
- Coutton C, Escoffier J, Martinez G, Arnoult C, Ray PF. Teratozoospermia: spotlight on the main genetic actors in the human. Hum Reprod Update 2015;21:455-485.
- Majzoub A, Agarwal A. Antioxidant therapy in idiopathic oligoasthenoteratozoospermia. Indian J Urol 2017;33:207-214.
- Dorostghoal M, Kazeminejad SR, Shahbazian N, Pourmehdi M, Jabbari A. Oxidative stress status and sperm DNA fragmentation in fertile and infertile men. Andrologia 2017;49:e12762.
- Osaka A, Okada H, Onozuka S, Tanaka T, Iwahata T, Shimomura Y, et al. Evaluation of the sperm DNA fragmentation index in infertile Japanese men by in-house flow cytometric analysis. Asian J Androl 2022;24:40-44.
- Giahi L, Mohammadmoradi S, Javidan A, Sadeghi MR. Nutritional modifications in male infertility: a systematic review covering 2 decades. Nutr Rev 2016;74:118-130.
- Du Plessis SS, Agarwal A, Halabi J, Tvrda E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J Assist Reprod Genet 2015;32:509-520.
- Harris ID, Fronczak C, Roth L, Meacham RB. Fertility and the aging male. Rev Urol 2011;13:e184-e190.
- Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M. Placebo-controlled double- blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil Steril 2005;84:662-671. PUBMED | CROSSREF
- Lewis SE, John Aitken R, Conner SJ, Iuliis GD, Evenson DP, Henkel R, et al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod Biomed Online 2013;27:325-337.
- Majzoub A, Agarwal A, Esteves SC. Antioxidants for elevated sperm DNA fragmentation: a mini review. Transl Androl Urol 2017;6:S649-S653.
- Ménézo YJ, Hazout A, Panteix G, Robert F, Rollet J, Cohen-Bacrie P, et al. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online 2007;14:418-421.
- Moskovtsev SI, Lecker I, Mullen JB, Jarvi K, Willis J, White J, et al. Cause-specific treatment in patients with high sperm DNA damage resulted in significant DNA improvement. Syst Biol Reprod Med 2009;55:109-115.
- Zhaku V, Beadini S, Beadini N, Amzai G. Oral antioxidants mitigate levels of malondialdehyde and protein carbonyl and improve semen parameters in men with oligoasthenozoospermia. Research Square. July 30, 2020. https://doi.org/10.21203/rs.3.rs-49942/v1.
- Micic S, Lalic N, Djordjevic D, Bojanic N, Bogavac-Stanojevic N, Busetto GM, et al. Double-blind, randomised, placebo-controlled trial on the effect of L-carnitine and L-acetylcarnitine on sperm parameters in men with idiopathic oligoasthenozoospermia. Andrologia 2019;51:e13267.
- Busetto GM, Agarwal A, Virmani A, Antonini G, Ragonesi G, Del Giudice F, et al. Effect of metabolic and antioxidant supplementation on sperm parameters in oligo-astheno-teratozoospermia, with and without varicocele: a double-blind placebo-controlled study. Andrologia 2018;50:el2927.
- Akmal M, Qadri JQ, Al-Waili NS, Thangal S, Haq A, Saloom KY. Improvement in human semen quality afier oral supplementation of vitamin C. J Med Food 2006;9:440-442.
- Tikkiwal M, Ajmera RL, Mathur NK. Effect of zinc administration on seminal zinc and fertility of oligospermic males. Indian J Physiol Pharmacol 1987;31:30-34.
- Ahmadi S, Bashiri R, Ghadiri-Anari A, Nadjarzadeh A. Antioxidant supplements and semen parameters: an evidence based review. Int J Reprod Biomed 2016;14:729-736.
- Vishvkarma R, Alahmar AT, Gupta G, Rajender S. Coenzyme Q10 effect on semen parameters: profound or meagre? Andrologia 2020;52:e13570.
- Blomberg Jensen M, Lawaetz JG, Petersen JH, Juul A, Jørgensen N. Effects of vitamin D supplementation on semen quality, reproductive hormones, and live birth rate: a randomized clinical trial. J Clin Endocrinol Metab 2018;103:870-881.
- Scaruffi P, Licata E, Maccarini E, Massarotti C, Bovis F, Sozzi F, et al. Oral antioxidant treatment of men significantly improves the reproductive outcome of IVF cycles. J Clin Med 2021;10:3254.
- Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev 2011;CD007411.

