Dr. Sarah K. Ahmed¹, Prof. Thomas J. Bennett², Dr. Li Mei Zhang³
1 Department of Public Health and Environmental Medicine, Northbridge University, London, UK.
2 School of Respiratory Sciences, Western Coast Medical College, Sydney, Australia
3 Division of Environmental Health, Tianjin Institute of Medical Research, Tianjin, China
* Corresponding Author: Dr. Ayesha Khan, Department of Global Health University of Nairobi P.O. Box 30197, Nairobi, Kenya; Email: [email protected]
Abstract
Airborne environmental pollutants pose a significant threat to respiratory well-being on a global scale. When pollutant concentrations surpass critical thresholds, inhalation can initiate respiratory impairments and intensify symptoms in individuals with established chronic respiratory disorders. Presently, more than 90% of the world’s population inhabits regions where air quality is deemed hazardous—resulting in health consequences that span from short-term airway inflammation to profound immune system disruptions. This narrative review offers a comprehensive overview of how environmental air pollution influences pulmonary health and physiological function, highlighting the biological mechanisms that contribute to the onset and advancement of chronic respiratory conditions.
Keywords: Airborne pollutants, Chronic respiratory disease, Pulmonary dysfunction, Environmental exposure, Immune modulation.
© The Author(s) 2025. Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third-party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
1. Introduction
Environmental air pollution is defined as the presence of undesirable chemical, physical, or biological substances in the atmosphere that pose risks to human health (Landrigan, 2017). Although the World Health Organisation (WHO) has established clear guidelines regarding the permissible annual and seasonal concentrations of key pollutants (WHO, n.d.), more than 90% of people globally currently live in urbanised and industrial regions where the air contains elevated levels of particulate matter (PM), ozone, sulfur oxides, and nitrogen oxides. Air pollution has become a major global health concern and is now acknowledged as a leading contributor to disease and premature death, with estimates linking it to approximately 4.2 million fatalities each year (WHO, n.d.). The respiratory system is especially susceptible to the adverse effects of polluted air, with recent epidemiological data associating exposure with a higher prevalence of acute respiratory infections, as well as increased risks of asthma, chronic obstructive pulmonary disease (COPD), and lung cancer (Gourd, 2022; Guo et al., 2018).
Assessing the negative consequences of air pollution on respiratory well-being often involves measuring lung function (Agustí et al., 2017), which includes detailed evaluations of airway dimensions, airflow resistance, lung capacity, and the efficiency of gas transfer at the alveolar-capillary interface (Sylvester et al., 2020). Both short-term and long-term exposures to environmental pollutants can adversely affect these physiological markers. The pollutants most frequently investigated include PM, ground-level ozone, and nitrogen dioxide (NO₂) (WHO, n.d.), which are widely distributed in the atmosphere, although their concentrations do not always reach levels that pose significant health threats. Nevertheless, the respiratory effects of air pollution are not uniformly experienced across populations. Outcomes can vary depending on total exposure and individual vulnerability. For instance, those at heightened risk include children, the elderly, individuals with pre-existing lung conditions, and populations living in poverty or under socio-economic strain (Aithal et al., 2023; Marmot & Bell, 2018).
Despite growing awareness of these risks, there remains a lack of comprehensive research examining how air pollutants affect lung function specifically in vulnerable groups. This narrative review aims to provide a current overview of how environmental air pollution impacts respiratory health and function and to explore the biological mechanisms involved in the development and worsening of chronic respiratory illnesses. Following the Scale for the Assessment of Narrative Review Articles (SANRA) (Baethge et al., 2019), relevant peer-reviewed literature published between 2018 and 2024 was sourced through PubMed and Google Scholar using broad keywords including “lung function,” “pulmonary function,” “air pollution,” “environmental pollution,” and “ambient pollution.”
2. Epidemiology Of Environmental Air Pollution
International air quality guidelines are largely informed by advanced modeling techniques that estimate the mass concentration of airborne pollutants and assess the corresponding health outcomes of exposure (Lelieveld et al., 2015). However, this methodology has limitations. Pollutants originating from diverse sources may differ significantly in chemical makeup, toxicity levels, and physical structure (Li et al., 2019). For example, recent research has highlighted more severe health consequences and higher mortality rates per unit increase in fine particulate matter (PM2.5) in Eastern Chinese urban centers such as Shanghai and Hangzhou, compared to other parts of China. This disparity exists despite the populations sharing similar environmental and socio-demographic characteristics. Likewise, data from Europe and the United States show higher mortality risk associated with the same increase in PM2.5 than observed in China (Chen et al., 2017). While elevated cardiovascular vulnerability in Western populations may partly account for this difference, accumulating evidence supports the idea that pollutant toxicity is not uniform worldwide (Li et al., 2019).
Environmental air typically contains a combination of harmless and harmful particulate matter originating from both natural and human-driven activities. Natural sources of airborne pollution include phenomena such as volcanic eruptions, dust and sandstorms, and wildfires (Li et al., 2019). In contrast, anthropogenic contributors encompass fuel combustion, agricultural practices, vehicular emissions, and industrial waste incineration. Among these, fuel combustion is the dominant contributor to air pollution on a global scale, accounting for approximately 85% of all PM2.5 emissions and nearly all oxidizing gases (OECD I, 2016). Air pollution patterns vary regionally due to differences in industrial activity, climatic conditions, and preferred energy sources (Lelieveld et al., 2015).
Although recognition of the detrimental health impacts of ambient air pollution has grown, mortality linked to poor air quality has increased by 66% over the last twenty years (Fuller et al., 2022). According to findings from the 2019 Lancet Commission on Planetary Health, over 4 million deaths were attributed to PM2.5, and another 370,000 to ozone exposure. High pollution levels have been associated with a surge in respiratory conditions such as infections of the respiratory tract (Asri et al., 2021), COPD (Guo et al., 2018), and lung cancer (Gourd, 2022). Additionally, asthma prevalence tends to be greater in highly polluted regions (Orellano et al., 2017). Short-term elevations in air pollution also correspond to worsening symptoms in individuals with chronic lung disease, leading to increased hospital visits and healthcare demands (Hunt et al., 2003; Johannson et al., 2014).
Importantly, the respiratory health burden from air pollution is not evenly shared across the globe. Nearly 75% of the world’s population resides in low- and middle-income nations, which also account for 90% of pollution-related deaths (Fuller et al., 2022). While research supports the health advantages of improving air quality (Gauderman et al., 2015), implementing such changes remains a significant hurdle in economically disadvantaged areas, where industrial growth often relies on polluting technologies (Moser & Satterthwaite, 2010). Additionally, the effects of climate change and extreme weather conditions exacerbate vulnerabilities in these regions (Keswani et al., 2022). As such, a coordinated global approach is necessary to address and reduce the disproportionate respiratory health burden in heavily polluted areas.
3. Pathophysiological Mechanisms Of Lung Function Impairment
Airborne environmental contaminants known to contribute to impaired pulmonary function include particulate matter (PM), nitrogen oxides (NOx), sulfur dioxide (SO₂), ozone (O₃), and volatile organic compounds. Each pollutant exerts distinct biological effects, influenced by factors such as dose, deposition site, and chemical toxicity (Mossman et al., 2007).
3.1 Factors impacting inhaled particle deposition
Due to its extensive surface area and continuous interaction with the external environment, the respiratory system is especially prone to damage from airborne pollutants (Cohen et al., 2017). The physiochemical properties of inhaled substances—such as solubility, size, and reactivity—govern their pattern of deposition and clearance, ultimately affecting how the lungs respond (see Table 1 for pollutant characteristics) (Weill, 2020). The location of deposition plays a major role in symptom manifestation: for example, pollutants deposited in the upper respiratory tract typically result in rhinitis, pharyngitis, and laryngitis; in the bronchi, they cause bronchitis or bronchopneumonia; while deeper penetration into lung parenchyma can result in pulmonary edema and pneumonia (Weill, 2020).
Table 1: Overview of common air pollutants that potentially contribute to the development of respiratory disease
Pollutant properties |
|||||
| Size | Solubility | Coagulability/reactivity | Density | Deposition | Respiratory disease |
| PM10 | ↑ | –b | ↓ | ↔c | Nasopharyngeal |
| PM2.5 | ↓ | –b | ↑ | ↔c | Bronchioalveolar |
| PM0.1 | ↓↓ | –b | ↑↑ | ↔c | Alveolar and pulmonary vascular |
| NO2 | –a | ↓ | ↔ | ↔ | Bronchioalveolar |
| SO2 | –a | ↑ | ↔ | ↑ | Nasopharyngeal |
| Ozone | –a | ↔ | ↑↑ | ↑ | Lower respiratory tract predominance |
The deposition of pollutants is mainly governed by particle size, shape, and solubility (Weill, 2020). Smaller and less dense particles with low molecular weight tend to remain airborne longer and more easily mix with other airborne substances (Krzeszowiak et al., 2016). Due to their high mobility and greater Brownian motion, such particles penetrate deeper into the respiratory tract. Highly water-soluble pollutants are more likely to deposit in the upper airways, interacting with mucus-laden airway linings (Weill, 2020). In contrast, poorly soluble substances tend to deposit deeper within the lungs and may persist in alveolar spaces for extended periods due to reduced clearance mechanisms (Lippmann et al., 1980). These deposition patterns are key in determining the type and severity of respiratory symptoms triggered by various pollutant mixtures.
3.2 Particulate Matter
Among all air pollutants, particulate matter is the most frequently linked to negative respiratory outcomes (Fuller et al., 2022). PM refers to airborne solid or liquid particles, often suspended alongside other pollutants. Based on aerodynamic diameter, PM is categorised into coarse (2.5–10 μm), fine (<2.5 μm), and ultrafine (<0.1 μm) particles. Coarse particles are generally filtered out by nasal hairs or deposited in the mucus-lined upper airway, although low-density variants may travel further into the respiratory tract (Krzeszowiak et al., 2016). Fine and ultrafine particles bypass upper defenses, with ultrafine particles reaching alveolar spaces that lack protective mucus layers (Lippmann et al., 1980).
PM exerts its pathogenic effects primarily through interactions with the respiratory epithelium and immune cells like alveolar macrophages, promoting oxidative stress and inflammation that damage lung tissue (Aghapour et al., 2022). The airway epithelium is composed of diverse cell types: ciliated and mucus-producing cells facilitate airway clearance, basal cells support epithelial integrity and regeneration, while resident immune cells monitor and respond to inhaled pathogens (Misiukiewicz-Stepien & Paplinska-Goryca, 2021). PM compromises epithelial integrity, particularly in individuals with existing airway diseases. Some particles directly generate reactive oxygen species (ROS) in the airway lining fluid (Lakey et al., 2016), while others cause dysfunction through intracellular signaling and mitochondrial injury (Aghapour et al., 2022). This results in excessive inflammation and structural remodeling—hallmarks of conditions like asthma and COPD (Iwanaga et al., 2013).
Although causality has not been definitively proven, multiple physiological links connect PM exposure with COPD onset and progression (Schikowski et al., 2014). Both acute and chronic PM exposure elevate systemic inflammatory markers—C-reactive protein, TNF-α, IL-6, and IL-8—all commonly seen in COPD (Li et al., 2022; Szalontai et al., 2021). Moreover, COPD involves a disrupted balance between proteases and anti-protease mechanisms, leading to tissue breakdown and airway remodeling. It is hypothesised that PM exposure heightens protease activity, damaging elastin fibers and contributing to the characteristic airway distortion seen in COPD (Ryu et al., 2022), although more evidence is needed.
PM also stimulates metaplasia and hyperplasia of mucus-producing cells in animal models (He et al., 2017; Montgomery et al., 2020). Inhaled particles impair mucociliary clearance by disrupting ciliary function, which is responsible for eliminating up to 90% of inhaled PM in healthy individuals (Krzeszowiak et al., 2016). This disruption contributes to persistent inflammation and recurrent infections. Furthermore, studies using diesel particle infusions into the trachea have demonstrated increased vagal nerve sensitivity, potentially triggering symptoms such as coughing and bronchospasm (Robinson et al., 2018), underscoring the diverse downstream impacts of PM inhalation.
An important property of PM is its ability to interact with other environmental molecules (see Table 1). PM2.5 particles remain suspended in air for prolonged periods and can bind to allergens, microbial components (e.g., fungal spores, bacterial polysaccharides, and endotoxins), and bioactive compounds (Joubert et al., 2020). The oxidative stress induced by these complexes depends on the nature of co-bound substances—particularly metals like copper, iron, zinc, and chromium (Mossman et al., 2007; Krzeszowiak et al., 2016). These combinations may result in both allergic responses, often IgE-mediated (Joubert et al., 2020), and non-allergic irritant effects (Burge et al., 2012).
A recent systematic review has found evidence linking PM—as well as ozone and NO₂—with an increased risk of pulmonary fibrosis (Harari et al., 2020). While the biological pathways remain under investigation, several plausible explanations exist. Ultrafine particles (<0.1 μm) can migrate to the lung interstitium between conducting airways and alveolar zones (Berend, 2016), potentially initiating fibrosis through persistent inflammation. In vitro studies suggest that PM exposure promotes epithelial–mesenchymal transition, proliferation of type II alveolar cells, and altered TGF-β signaling—hallmarks of fibrotic processes (Johannson et al., 2015). Additionally, the release of cytokines like IL-4 and IL-13, and ROS from alveolar macrophages and epithelial cells, may provoke epigenetic changes and telomere shortening—mechanisms commonly associated with repeated epithelial injury and faulty repair in interstitial lung diseases (Wei et al., 2024).
3.3 Ozone
Tropospheric ozone is produced through photochemical reactions that occur when sunlight interacts with precursor pollutants such as nitrogen oxides (NOx) and volatile organic compounds (VOCs) (Guarnieri & Balmes, 2014). VOCs refer to organic chemicals that contain at least one carbon-hydrogen bond and evaporate easily under standard conditions (Rumchev et al., 2007). Ozone serves as a strong oxidising compound capable of reacting with lipids, proteins, fatty acids, and surface molecules within the respiratory tract, disrupting the epithelial barrier and compromising its integrity (Schikowski et al., 2014). Consequently, elevated ozone levels in the environment are linked to worsening of multiple respiratory illnesses (Duan et al., 2020; Harari et al., 2020; Shin et al., 2020). Due to its intermediate solubility, ozone is able to irritate various parts of the respiratory tract, including the upper airways, bronchi, and pulmonary parenchyma (Weill, 2020). Ambient concentrations of ozone ranging between 0.2 and 0.6 ppm have been associated with indicators of cellular injury in bronchoalveolar lavage fluid (Aris et al., 1993), an increased likelihood of allergic sensitisation leading to the onset of asthma in paediatric populations (Kim et al., 2011), and a heightened airway responsiveness to bronchial challenge tests (Kehrl et al., 1999; Seltzer et al., 1986).
Evidence from several studies indicates that ozone exposure may promote eosinophilic inflammation (Aris et al., 1993; Seltzer et al., 1986), though these findings are most evident in individuals with pre-existing asthma, which complicates attempts to infer a causal relationship. Even short-term exposure to relatively low concentrations of ozone—less than 70 ppb over an eight-hour average—has been correlated with reductions in lung function among children and elderly individuals (Holm & Balmes, 2022). However, the influence of ozone on pulmonary performance in the general adult population remains somewhat uncertain, especially at concentrations between 1 and 42 ppb. Any observed reductions in forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) tend to be minor and become more significant with advancing age or repeated exposure over several days (Holm & Balmes, 2022). Further investigation is essential to isolate the specific effects of ozone in ambient settings, considering potential confounders such as smoking status and underlying asthma.
3.4 Oxidizing gases
Reactive gases such as nitrogen dioxide (NO2) and sulfur dioxide (SO2) are prevalent components of atmospheric air pollution and exert dose-dependent effects on respiratory tissues. NO2, being relatively insoluble, can travel deep into the distal alveoli (Weill, 2020), while SO2, which is highly water-soluble, typically affects the upper respiratory tract upon exposure. These gases, like ozone and particulate matter (PM), cause damage to the airway lining by stimulating the production of reactive oxygen species (ROS) and inflammatory cytokines, with well-documented links to the exacerbation of respiratory conditions (Faustini et al., 2014; Koenig et al., 1985; Strand et al., 1997; Tunnicliffe et al., 1994).
High exposure to NO2 has been shown to cause chemical pneumonitis, which, if not promptly treated, may progress to fibrosis of lung tissue (Bauer et al., 1998). The respiratory consequences of chronic exposure to lower NO2 concentrations are subtler. Some reports suggest minimal effects when particulate matter is considered as a co-exposure (Faustini et al., 2014; Robertson et al., 1984). NO2 tends to accumulate in regions with heavy vehicular traffic, and several investigations have found that exposure at these sites can influence small airway performance (Robinson et al., 2022; Schultz et al., 2016). Still, the overall effect on standard lung function measures such as spirometry in adults is often negligible (Kerr et al., 1979; Robertson et al., 1984). In contrast, children appear more vulnerable, with even minor NO2 exposure linked to obstructive patterns in lung function (Moshammer et al., 2006).
The epithelial injury caused by NO2 promotes an inflammatory cascade involving various signalling molecules. In particular, upregulation of cytokines such as IL-4, IL-5, and IL-13 contributes to increased eosinophilic inflammation in the airways (Bevelander et al., 2007), which can be monitored non-invasively via fractional exhaled nitric oxide (FeNO) levels (Chung, 2021). Studies have demonstrated that concurrent exposure to NO2 and fine particulate matter (PM2.5) is associated with elevated FeNO among school-aged children living in polluted urban settings (Zhang et al., 2021). Moreover, NO2 exposure heightens epithelial permeability and responsiveness to allergens, with research indicating that household NO2 levels correlate with greater sensitisation to allergens like house dust mites in asthmatic individuals (Tunnicliffe et al., 1994). Brief periods of exposure to NO2 concentrations between 400 and 490 ppb can also lead to increased bronchial responsiveness during allergen inhalation, supporting its contributory role in the development or worsening of allergic asthma (Devalia et al., 1994; Strand et al., 1997).
SO2, by contrast, produces effects that are most evident in the upper airway due to its high solubility. Inhalation through the mouth of SO2 concentrations greater than 5 ppb causes bronchoconstriction in healthy individuals, while more subtle declines in peak expiratory flow (PEF) have been documented at levels above 1 ppb (Johns & Linn, 2011). For individuals with asthma, the health risks associated with SO2 exposure are more pronounced, with increased rates of emergency healthcare utilisation following acute episodes (Bethel et al., 1984; Koenig et al., 1985). Epidemiological data from urban areas further supports its role in respiratory morbidity, as mortality rates rise significantly for every 10 μg/m³ increment in ambient SO2 concentration (Orellano et al., 2017), indicating its likely involvement in both the frequency and severity of disease flare-ups.
4. Quantifying The Impact Of Air Pollution On Respiratory Function
The detrimental effects of air pollution on the lungs can be measured through various techniques that assess pulmonary function and airway inflammation. Among the available physiological evaluations, spirometry and fractional exhaled nitric oxide (FeNO) are most commonly employed to gauge the respiratory consequences of air pollutant exposure. Spirometry offers quantifiable insights into dynamic lung volumes and airway patency (Sylvester et al., 2020), while impulse oscillometry—a non-effort-dependent method evaluating respiratory impedance—may offer increased sensitivity to subtle pollution-induced changes (Schultz et al., 2016). Both acute and long-term exposure to particulate matter (PM) has been linked with measurable reductions in key spirometric parameters such as forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) (Edginton et al., 2019). For instance, a 10 μg/m³ rise in short-term PM2.5 or chronic PM10 exposure corresponds with a yearly decline of approximately 7–9 mL in FEV1.
Although these reductions may appear minor and align with natural age-related deterioration, they take on more significance in low-income countries, where PM concentration fluctuations can reach up to 100 μg/m³ weekly (Hao et al., 2017), thus potentially accelerating pulmonary decline. However, spirometry has notable limitations in its sensitivity to detect early or subtle respiratory impairments due to air pollution (Kim et al., 2020; Schultz et al., 2016). The consistency of spirometric testing and duration of follow-up are critical factors in accurately capturing pollution-related pulmonary changes over time (Hnizdo et al., 2005), and both can vary widely depending on regional testing practices and healthcare resources.
The type of respiratory pathology triggered by pollution also influences the sensitivity of spirometry. In diseases focused on airway inflammation, conventional spirometry may overlook early changes in peripheral airways (Kim et al., 2020). Similarly, in interstitial or fibrotic lung diseases, spirometric values may remain within normal ranges, even when gas exchange begins to deteriorate before measurable volume loss occurs (Sylvester et al., 2021). Additionally, some investigations have relied on suboptimal measures such as the forced expiratory flow at 25% to 75% of FVC (FEF25-75) to detect small-to-medium airway involvement (Berend, 2016). However, the accuracy of FEF25-75 is questionable due to its high variability and dependence on precise FVC and peak expiratory flow for reliability.
-
Susceptible/High Risk Populations
The intensified inflammatory response triggered by inhaled air pollutants is often compounded by weakened antioxidant defense mechanisms (Liu et al., 2019). People with inherently lower antioxidant reserves or genetic variations that impair antioxidant efficiency (Chen et al., 2007) are more prone to oxidative stress and pulmonary function decline when exposed to polluted air (Bowatte et al., 2017). Conditions characterised by excessive mucus production and recurrent bacterial colonisation tend to exhibit a complex inflammatory profile, which may worsen disease progression (Aghapour et al., 2022; Dickerhof et al., 2017). Prolonged exposure to air pollutants may provoke or accelerate the onset of chronic respiratory illnesses due to this persistent inflammatory state (Altman et al., 2023; Johannson et al., 2014).
5.1 Pediatric Populations
Children are particularly sensitive to air pollution due to their elevated respiratory rates and higher ventilation relative to body weight (Aithal et al., 2023). Compared to adults, children’s nasal passages are less efficient in filtering airborne particles, resulting in an increased likelihood of larger particulates depositing in the upper respiratory tract (Bateson & Schwartz, 2007). In addition, their immune systems—both innate and adaptive—are still maturing, rendering them more susceptible to respiratory complications. Even minimal exposure to pollutants has been linked with heightened risks of developing allergic sensitisations and childhood asthma (Gasana et al., 2012; Olsson et al., 2021). Many chronic respiratory conditions manifest during childhood, and lung function in early adulthood is a strong determinant of respiratory health in later life (Bush, 2016). Longitudinal cohort studies indicate that early exposure to particulate matter is associated with diminished tidal volume, elevated respiratory rates, and an increased lung clearance index within the first year of life (Gray et al., 2017; Lee et al., 2019), as well as elevated resistance in the peripheral airways (Robinson et al., 2022; Schultz et al., 2016).
5.2 Older Adults
The cumulative exposure to environmental air pollution increases across the lifespan—alongside social, household, and occupational risk factors (Sandström et al., 2003). An age-associated loss of lung function occurs after 30 years of age due to reduced lung elasticity and diaphragm strength (Agustí et al., 2017). In the distal airways, airflow obstruction and ventilation heterogeneity can occur as a consequence of reduced lung elasticity occurring with age, which increases particle deposition in peripheral airways (Segal et al., 2002). This is worsened by slowing of mucociliary clearance and cough; two factors essential to airway clearance of inhaled particles (Bailey, 2022). The antioxidant defense systems, which protect against oxidative pollutants, are also impaired with age (Liu et al., 2019), increasing susceptibility to exaggerated inflammatory responses and infection. As elderly individuals have lower levels of ventilatory reserve in absolute terms as well as co-morbidities such as frailty, pollution-related exacerbations have the potential to have life-altering effects.
The cumulative burden of air pollutant exposure rises with age, compounded by socio-environmental, occupational, and household factors (Sandström et al., 2003). Lung function naturally declines after the age of 30, primarily due to the deterioration of pulmonary elasticity and weakening of the diaphragm (Agustí et al., 2017). In the distal airways, this age-related loss of elasticity can lead to obstructed airflow and uneven ventilation, encouraging deeper particle deposition (Segal et al., 2002). Ageing also slows mucociliary clearance and weakens the cough reflex—both essential for expelling inhaled particles (Bailey, 2022). Moreover, the antioxidant systems that defend against oxidative insults decline over time (Liu et al., 2019), making older individuals more prone to heightened inflammatory responses and respiratory infections. Given their reduced ventilatory capacity and common coexisting conditions such as frailty, pollutant-induced respiratory events can significantly impact quality of life in the elderly.
5.3 Pregnant Women
Both the pregnant mother and developing fetus are increasingly vulnerable to the effects of polluted air, especially from intrauterine exposure. During the first trimester, maternal minute ventilation rises by nearly 48% and remains elevated due to increased metabolic demands during pregnancy (LoMauro & Aliverti, 2015). This increase is mainly attributed to a rise in tidal volume, which reduces anatomical dead space and enables pollutants to penetrate deeper into the lungs. Fetal lung development continues until around the 36th week of gestation, and ultrafine particles capable of entering the bloodstream may potentially affect fetal development even before lung maturation (Bongaerts et al., 2022).
The harmful effects of environmental toxins like cigarette smoke on fetal health are well established (O’Shaughnessy et al., 2011). Similarly, combustion-related airborne particulates are increasingly linked to poor neonatal outcomes (LoMauro & Aliverti, 2015). These outcomes include increased risk of premature birth (Mendola et al., 2016), reduced birth weight (Fleisch et al., 2015), and hindered postnatal lung development (Latzin et al., 2009). Impulse oscillometry has proven valuable in identifying pollution-induced alterations in the respiratory systems of neonates, with evidence pointing to increased resistance in both small and large airways following exposure to household or vehicular emissions (Agyapong et al., 2023; Dutta et al., 2021). Consequently, the respiratory consequences of air pollution may originate prenatally and continue to influence health across the lifespan.
5.4 Athletic Populations
Athletes—both elite and recreational—face considerable exposure to air pollution during training and competitions, particularly those held outdoors. As physical exertion increases, so does ventilation to meet metabolic needs, first by expanding tidal volume and then by raising respiratory frequency at peak intensity (Pritchard et al., 2021). During intense activity, ventilation may increase 20 to 30 times above resting levels in elite performers (Price et al., 2019). When airflow surpasses 30 L/min, breathing transitions from nasal to combined oral-nasal inhalation, permitting greater volumes of unfiltered, unconditioned air—including pollutants and allergens—into the lower respiratory tract (Price, Walsted, et al., 2022).
It is estimated that one in five athletes display signs of lower airway dysfunction, demonstrated by a drop of more than 10% in FEV1 following exertion (Price, Sewry, et al., 2022). Repetitive high-intensity exercise under polluted conditions may result in cumulative airway trauma, potentially triggering chronic respiratory symptoms (Kippelen & Anderson, 2012). Based on these findings, some have proposed that airway dysfunction linked to elite sports participation should be recognised as an “occupational lung disease,” and athletes should receive occupational-level health protection due to their repeated exposure (Price et al., 2013).
5.5 Patients with Existing Respiratory Disorders
In individuals with pre-existing respiratory issues, air pollution can act as a catalyst for symptom exacerbation by disrupting the epithelial barrier and activating oxidative and inflammatory cascades (Aghapour et al., 2022). Pollutants may provoke inflammation, airway narrowing, and enhanced airway sensitivity and permeability, especially to irritants and allergens (Guarnieri & Balmes, 2014; Johannson et al., 2015). For those with limited lung function and reduced respiratory reserve, these pollutant-driven aggravations significantly elevate risks of hospitalisation and mortality (Agustí et al., 2017). Epidemiological studies have consistently shown strong links between air pollution and increased medical visits for respiratory issues across various conditions (Perez et al., 2013).
Additionally, respiratory diseases often follow socioeconomic patterns—individuals living in poverty or disadvantaged neighborhoods are disproportionately affected by both air pollution and chronic lung disease, making it challenging to disentangle environmental and social determinants of respiratory health (Marmot & Bell, 2018).
6. Summary: Current Perspectives And Future Challenges
Environmental air pollution continues to pose a significant threat to global respiratory health. This review has outlined how widespread airborne pollutants—including particulate matter (PM), ozone, and oxidising gases—can trigger respiratory inflammation, impair ciliary function, contribute to airway structural changes, and elicit symptoms such as coughing. When pollutant concentrations surpass critical thresholds, they may cause respiratory dysfunction or aggravate existing chronic respiratory conditions (see Figure 1). Crucially, mitigation measures targeting air pollution have been associated with improved respiratory outcomes (Gauderman et al., 2015). As such, there is a pressing need for coordinated global action to decrease pollutant emissions, with policy frameworks that account for the unequal burden borne by certain populations and regions.
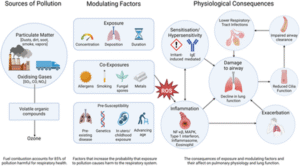
With regard to measuring the respiratory consequences of environmental pollution, conventional spirometry may not offer sufficient sensitivity to detect early pathological changes. Recent investigations have employed impulse oscillometry to assess airway resistance and reactance (Cottee et al., 2020; Kim et al., 2020; Postma et al., 2019), enabling the identification of more nuanced signs of respiratory compromise, particularly involving the small airways. Additionally, newer diagnostic modalities such as hyperpolarised magnetic resonance imaging and exhaled breath analysis (Stewart et al., 2022) have demonstrated potential in evaluating respiratory symptoms when traditional lung function tests are inconclusive.
Looking ahead, future studies should aim to integrate these advanced diagnostic tools into large-scale epidemiological research. Such efforts would help establish more precise relationships between specific air pollutants and respiratory effects. Ultimately, this could facilitate the creation of targeted preventative measures designed to protect individuals and communities from pollution-related respiratory illness, supporting public health on both a micro and macro scale.
References
Aghapour, M., Ubags, N. D., Bruder, D., Hiemstra, P. S., Sidhaye, V., Rezaee, F., & Heijink, I. H. (2022). Role of air pollutants in airway epithelial barrier dysfunction in asthma and COPD. European Respiratory Review, 31(163), 210112.
Agustí, A., Noell, G., Brugada, J., & Faner, R. (2017). Lung function in early adulthood and health in later life: A transgenerational cohort analysis. The Lancet Respiratory Medicine, 5(12), 935–945.
Agyapong, P. D., Jack, D., Kaali, S., Colicino, E., Mujtaba, M. N., Chillrud, S. N., Osei, M., Gennings, C., Agyei, O., Kinney, P. L., Kwarteng, A., Perzanowski, M., Dwommoh Prah, R. K., Tawiah, T., Asante, K. P., & Lee, A. G. (2023). Household air pollution and child lung function: The Ghana randomized air pollution and health study. American Journal of Respiratory and Critical Care Medicine, 209, 716–726.
Aithal, S. S., Sachdeva, I., & Kurmi, O. P. (2023). Air quality and respiratory health in children. Breathe, 19(2), 230040.
Altman, M. C., Kattan, M., O’Connor, G. T., Murphy, R. C., Whalen, E., LeBeau, P., Calatroni, A., Gill, M. A., Gruchalla, R. S., Liu, A. H., Lovinsky-Desir, S., Pongracic, J. A., Kercsmar, C. M., Khurana Hershey, G. K., Zoratti, E. M., Teach, S. J., Bacharier, L. B., Wheatley, L. M., Sigelman, S. M., … National Institute of Allergy and Infectious Disease’s Inner City Asthma Consortium. (2023). Associations between outdoor air pollutants and non-viral asthma exacerbations and airway inflammatory responses in children and adolescents living in urban areas in the USA: A retrospective secondary analysis. The Lancet Planetary Health, 7(1), e33–e44.
Aris, R. M., Christian, D., Hearne, P. Q., Kerr, K., Finkbeiner, W. E., & Balmes, J. R. (1993). Ozone-induced airway inflammation in human subjects as determined by airway lavage and biopsy. The American Review of Respiratory Disease, 148, 1363–1372.
Asri, A. K., Pan, W.-C., Lee, H.-Y., Su, H.-J., Wu, C.-D., & Spengler, J. D. (2021). Spatial patterns of lower respiratory tract infections and their association with fine particulate matter. Scientific Reports, 11(1), 4866.
Baethge, C., Goldbeck-Wood, S., & Mertens, S. (2019). SANRA—A scale for the quality assessment of narrative review articles. Research Integrity and Peer Review, 4, 1–7.
Bailey, K. L. (2022). Aging diminishes Mucociliary clearance of the lung. Advances in Geriatric Medicine and Research, 4(2), 1–12.
Bateson, T. F., & Schwartz, J. (2007). Children’s response to air pollutants. Journal of Toxicology and Environmental Health, Part A, 71(3), 238–243.
Bauer, U., Berg, D., Kohn, M. A., Meriwether, R. A., & Nickle, R. A. (1998). Acute effects of nitrogen dioxide after accidental release. Public Health Reports, 113(1), 62.
Berend, N. (2016). Contribution of air pollution to COPD and small airway dysfunction. Respirology, 21(2), 237–244.
Bethel, R. A., Sheppard, D., Epstein, J., Tam, E., Nadel, J., & Boushey, H. A. (1984). Interaction of sulfur dioxide and dry cold air in causing bronchoconstriction in asthmatic subjects. Journal of Applied Physiology, 57(2), 419–423.
Bevelander, M., Mayette, J., Whittaker, L. A., Paveglio, S. A., Jones, C. C., Robbins, J., Hemenway, D., Akira, S., Uematsu, S., & Poynter, M. E. (2007). Nitrogen dioxide promotes allergic sensitization to inhaled antigen. The Journal of Immunology, 179(6), 3680–3688.
Bongaerts, E., Lecante, L. L., Bové, H., Roeffaers, M. B., Ameloot, M., Fowler, P. A., & Nawrot, T. S. (2022). Maternal exposure to ambient black carbon particles and their presence in maternal and fetal circulation and organs: An analysis of two independent population-based observational studies. The Lancet Planetary Health, 6(10), e804–e811.
Bowatte, G., Erbas, B., Lodge, C. J., Knibbs, L. D., Gurrin, L. C., Marks, G. B., Thomas, P. S., Johns, D. P., Giles, G. G., Hui, J., Dennekamp, M., Perret, J. L., Abramson, M. J., Walters, E. H., Matheson, M. C., & Dharmage, S. C. (2017). Traffic-related air pollution exposure over a 5-year period is associated with increased risk of asthma and poor lung function in middle age. The European Respiratory Journal, 50(4), 1602357.
Burge, P., Moore, V., & Robertson, A. (2012). Sensitization and irritant-induced occupational asthma with latency are clinically indistinguishable. Occupational Medicine, 62(2), 129–133.
Bush, A. (2016). Lung development and aging. Annals of the American Thoracic Society, 13(Supplement 5), S438–S446.
Chen, C., Arjomandi, M., Tager, I. B., Holland, N., & Balmes, J. R. (2007). Effects of antioxidant enzyme polymorphisms on ozone-induced lung function changes. The European Respiratory Journal, 30(4), 677–683.
Chen, R., Yin, P., Meng, X., Liu, C., Wang, L., Xu, X., Ross, J. A., Tse, L. A., Zhao, Z., Kan, H., & Zhou, M. (2017). Fine particulate air pollution and daily mortality. A nationwide analysis in 272 Chinese cities. American Journal of Respiratory and Critical Care Medicine, 196(1), 73–81.
Choi, J., Sim, J. K., Oh, J. Y., Lee, Y. S., Hur, G. Y., Lee, S. Y., Shim, J. J., Moon, J. Y., & Min, K. H. (2020). Relationship between particulate matter (PM10) and airway inflammation measured with exhaled nitric oxide test in Seoul, Korea. Canadian Respiratory Journal, 2020(1), 1823405.
Chung, K. F. (2021). Increasing utility of FeNO as a biomarker of type-2 inflammation in severe asthma. The Lancet Respiratory Medicine, 9(10), 1083–1084.
Cohen, A. J., Brauer, M., Burnett, R., Anderson, H. R., Frostad, J., Estep, K., Balakrishnan, K., Brunekreef, B., Dandona, L., Dandona, R., Feigin, V., Freedman, G., Hubbell, B., Jobling, A., Kan, H., Knibbs, L., Liu, Y., Martin, R., Morawska, L., … Forouzanfar, M. H. (2017). Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the global burden of diseases study 2015. The Lancet, 389(10082), 1907–1918.
Cottee, A. M., Seccombe, L. M., Thamrin, C., King, G. G., Peters, M. J., & Farah, C. S. (2020). Bronchodilator response assessed by the forced oscillation technique identifies poor asthma control with greater sensitivity than spirometry. Chest, 157(6), 1435–1441.
Devalia, J., Rusznak, C., Herdman, M., Trigg, C., Davies, R., & Tarraf, H. (1994). Effect of nitrogen dioxide and sulphur dioxide on airway response of mild asthmatic patients to allergen inhalation. The Lancet, 344(8938), 1668–1671.
Dickerhof, N., Pearson, J. F., Hoskin, T. S., Berry, L. J., Turner, R., Sly, P. D., Kettle, A. J., & AREST CF. (2017). Oxidative stress in early cystic fibrosis lung disease is exacerbated by airway glutathione deficiency. Free Radical Biology & Medicine, 113, 236–243.
Doiron, D., de Hoogh, K., Probst-Hensch, N., Fortier, I., Cai, Y., De Matteis, S., & Hansell, A. L. (2019). Air pollution, lung function and COPD: Results from the population-based UK biobank study. The European Respiratory Journal, 54(1), 1802140.
Duan, R.-R., Hao, K., & Yang, T. (2020). Air pollution and chronic obstructive pulmonary disease. Chronic Diseases and Translational Medicine, 6(4), 260–269.
Dutta, A., Alaka, M., Ibigbami, T., Adepoju, D., Adekunle, S., Olamijulo, J., Adedokun, B., Deji-Abiodun, O., Chartier, R., Ojengbede, O., & Olopade, C. O. (2021). Impact of prenatal and postnatal household air pollution exposure on lung function of 2-year old Nigerian children by oscillometry. The Science of the Total Environment, 755, 143419.
Edginton, S., O’Sullivan, D. E., King, W., & Lougheed, M. D. (2019). Effect of outdoor particulate air pollution on FEV1 in healthy adults: A systematic review and meta-analysis. Occupational and Environmental Medicine, 76(8), 583–591.
Faustini, A., Rapp, R., & Forastiere, F. (2014). Nitrogen dioxide and mortality: Review and meta-analysis of long-term studies. The European Respiratory Journal, 44(3), 744–753.
Fleisch, A. F., Rifas-Shiman, S. L., Koutrakis, P., Schwartz, J. D., Kloog, I., Melly, S., Coull, B. A., Zanobetti, A., Gillman, M. W., Gold, D. R., & Oken, E. (2015). Prenatal exposure to traffic pollution: Associations with reduced fetal growth and rapid infant weight gain. Epidemiology (Cambridge, Mass.), 26(1), 43–50.
Fuller, R., Landrigan, P. J., Balakrishnan, K., Bathan, G., Bose-O’Reilly, S., Brauer, M., Caravanos, J., Chiles, T., Cohen, A., Corra, L., Cropper, M., Ferraro, G., Hanna, J., Hanrahan, D., Hu, H., Hunter, D., Janata, G., Kupka, R., Lanphear, B., … Yan, C. (2022). Pollution and health: A progress update. The Lancet Planetary Health, 6(6), e535–e547.
Gasana, J., Dillikar, D., Mendy, A., Forno, E., & Vieira, E. R. (2012). Motor vehicle air pollution and asthma in children: A meta-analysis. Environmental Research, 117, 36–45.
Gauderman, W. J., Urman, R., Avol, E., Berhane, K., McConnell, R., Rappaport, E., Chang, R., Lurmann, F., & Gilliland, F. (2015). Association of improved air quality with lung development in children. The New England Journal of Medicine, 372(10), 905–913.
Gourd, E. (2022). New evidence that air pollution contributes substantially to lung cancer. The Lancet Oncology, 23(10), e448.
Gray, D. M., Turkovic, L., Willemse, L., Visagie, A., Vanker, A., Stein, D. J., Sly, P. D., Hall, G. L., & Zar, H. J. (2017). Lung function in African infants in the Drakenstein child health study. Impact of lower respiratory tract illness. American Journal of Respiratory and Critical Care Medicine, 195(2), 212–220.
Guarnieri, M., & Balmes, J. R. (2014). Outdoor air pollution and asthma. The Lancet, 383(9928), 1581–1592.
Guo, C., Zhang, Z., Lau, A. K., Lin, C. Q., Chuang, Y. C., Chan, J., Jiang, W. K., Tam, T., Yeoh, E. K., Chan, T. C., Chang, L. Y., & Lao, X. Q. (2018). Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: A longitudinal, cohort study. The Lancet Planetary Health, 2(3), e114–e125.
Habre, R., Moshier, E., Castro, W., Nath, A., Grunin, A., Rohr, A., Godbold, J., Schachter, N., Kattan, M., Coull, B., & Koutrakis, P. (2014). The effects of PM2. 5 and its components from indoor and outdoor sources on cough and wheeze symptoms in asthmatic children. Journal of Exposure Science & Environmental Epidemiology, 24(4), 380–387.
Hao, Y., Zhang, G., Han, B., Xu, X., Feng, N., Li, Y., Wang, W., Kan, H., Bai, Z., Zhu, Y., Au, W., & Xia, Z. L. (2017). Prospective evaluation of respiratory health benefits from reduced exposure to airborne particulate matter. International Journal of Environmental Health Research, 27(2), 126–135.
Harari, S., Raghu, G., Caminati, A., Cruciani, M., Franchini, M., & Mannucci, P. (2020). Fibrotic interstitial lung diseases and air pollution: A systematic literature review. European Respiratory Review, 29(157), 200093.
He, F., Liao, B., Pu, J., Li, C., Zheng, M., Huang, L., Zhou, Y., Zhao, D., Li, B., & Ran, P. (2017). Exposure to ambient particulate matter induced COPD in a rat model and a description of the underlying mechanism. Scientific Reports, 7(1), 45666.
Hnizdo, E., Yu, L., Freyder, L., Attfield, M., Lefante, J., & Glindmeyer, H. (2005). The precision of longitudinal lung function measurements: Monitoring and interpretation. Occupational and Environmental Medicine, 62(10), 695–701.
Holm, S. M., & Balmes, J. R. (2022). Systematic review of ozone effects on human lung function, 2013 through 2020. Chest, 161(1), 190–201.
Huang, F., Pan, B., Wu, J., Chen, E., & Chen, L. (2017). Relationship between exposure to PM2. 5 and lung cancer incidence and mortality: A meta-analysis. Oncotarget, 8(26), 43322.
Hunt, A., Abraham, J. L., Judson, B., & Berry, C. L. (2003). Toxicologic and epidemiologic clues from the characterization of the 1952 London smog fine particulate matter in archival autopsy lung tissues. Environmental Health Perspectives, 111(9), 1209–1214.
Iwanaga, K., Elliott, M. S., Vedal, S., & Debley, J. S. (2013). Urban particulate matter induces pro-remodeling factors by airway epithelial cells from healthy and asthmatic children. Inhalation Toxicology, 25(12), 653–660.
Johannson, K. A., Balmes, J. R., & Collard, H. R. (2015). Air pollution exposure: A novel environmental risk factor for interstitial lung disease? Chest, 147(4), 1161–1167.
Johannson, K. A., Vittinghoff, E., Lee, K., Balmes, J. R., Ji, W., Kaplan, G. G., Kim, D. S., & Collard, H. R. (2014). Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. The European Respiratory Journal, 43(4), 1124–1131.
Johns, D. O., & Linn, W. S. (2011). A review of controlled human SO2 exposure studies contributing to the US EPA integrated science assessment for sulfur oxides. Inhalation Toxicology, 23(1), 33–43.
Joubert, A. I., Geppert, M., Johnson, L., Mills-Goodlet, R., Michelini, S., Korotchenko, E., Duschl, A., Weiss, R., Horejs-Höck, J., & Himly, M. (2020). Mechanisms of particles in sensitization, effector function and therapy of allergic disease. Frontiers in Immunology, 11, 1334.
Kehrl, H. R., Peden, D. B., Ball, B., Folinsbee, L. J., & Horstman, D. (1999). Increased specific airway reactivity of persons with mild allergic asthma after 7.6 hours of exposure to 0.16 ppm ozone. The Journal of Allergy and Clinical Immunology, 104(6), 1198–1204.
Kerr, H. D., Kulle, T. J., McIlhany, M. L., & Swidersky, P. (1979). Effects of nitrogen dioxide on pulmonary function in human subjects: An environmental chamber study. Environmental Research, 19(2), 392–404.
Keswani, A., Akselrod, H., & Anenberg, S. C. (2022). Health and clinical impacts of air pollution and linkages with climate change. NEJM Evidence, 1(7), EVIDra2200068.
Kim, B.-J., Kwon, J.-W., Seo, J.-H., Kim, H.-B., Lee, S.-Y., Park, K.-S., Yu, J., Kim, H. C., Leem, J. H., Sakong, J., Kim, S. Y., Lee, C. G., Kang, D. M., Ha, M., Hong, Y. C., Kwon, H. J., & Hong, S. J. (2011). Association of ozone exposure with asthma, allergic rhinitis, and allergic sensitization. Annals of Allergy, Asthma & Immunology, 107(3), 214–219. e1.
Kim, S.-R., Park, K. H., Son, N.-H., Moon, J., Park, H. J., Kim, K., Park, J. W., & Lee, J. H. (2020). Application of impulse oscillometry in adult asthmatic patients with preserved lung function. Allergy, Asthma & Immunology Research, 12(5), 832–843.
Kippelen, P., & Anderson, S. D. (2012). Airway injury during high-level exercise. British Journal of Sports Medicine, 46(6), 385–390.
Koenig, J. Q., Morgan, M. S., Horike, M., & Pierson, W. E. (1985). The effects of sulfur oxides on nasal and lung function in adolescents with extrinsic asthma. The Journal of Allergy and Clinical Immunology, 76(6), 813–818.
Krzeszowiak, J., Stefanow, D., & Pawlas, K. (2016). The impact of particulate matter (PM) and nitric oxides (NOx) on human health and an analysis of selected sources accounting for their emission in Poland. Medycyna Środowiskowa-Environmental Medicine, 19(3), 7–15.
Lakey, P. S., Berkemeier, T., Tong, H., Arangio, A. M., Lucas, K., Pöschl, U., & Shiraiwa, M. (2016). Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Scientific Reports, 6(1), 32916.
Landrigan, P. J. (2017). Air pollution and health. The Lancet Public Health, 2(1), e4–e5.
Latzin, P., Röösli, M., Huss, A., Kuehni, C. E., & Frey, U. (2009). Air pollution during pregnancy and lung function in newborns: A birth cohort study. The European Respiratory Journal, 33(3), 594–603.
Lee, A. G., Kaali, S., Quinn, A., Delimini, R., Burkart, K., Opoku-Mensah, J., Wylie, B. J., Yawson, A. K., Kinney, P. L., Ae-Ngibise, K. A., Chillrud, S., Jack, D., & Asante, K. P. (2019). Prenatal household air pollution is associated with impaired infant lung function with sex-specific effects. Evidence from GRAPHS, a cluster randomized cookstove intervention trial. American Journal of Respiratory and Critical Care Medicine, 199(6), 738–746.
Lelieveld, J., Evans, J. S., Fnais, M., Giannadaki, D., & Pozzer, A. (2015). The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature, 525(7569), 367–371.
Li, T., Yu, Y., Sun, Z., & Duan, J. (2022). A comprehensive understanding of ambient particulate matter and its components on the adverse health effects based from epidemiological and laboratory evidence. Particle and Fibre Toxicology, 19(1), 67.
Li, X., Jin, L., & Kan, H. (2019). Air pollution: A global problem needs local fixes. Nature, 570(7762), 437–439.
Lin, L., Li, T., Sun, M., Liang, Q., Ma, Y., Wang, F., Duan, J., & Sun, Z. (2021). Effect of particulate matter exposure on the prevalence of allergic rhinitis in children: A systematic review and meta-analysis. Chemosphere, 268, 128841.
Lippmann, M., Yeates, D., & Albert, R. (1980). Deposition, retention, and clearance of inhaled particles. Occupational and Environmental Medicine, 37(4), 337–362.
Liu, X., Wang, J., Fan, Y., Xu, Y., Xie, M., Yuan, Y., Li, H., & Qian, X. (2019). Particulate matter exposure history affects antioxidant defense response of mouse lung to haze episodes. Environmental Science & Technology, 53(16), 9789–9799.
LoMauro, A., & Aliverti, A. (2015). Respiratory physiology of pregnancy: Physiology masterclass. Breathe, 11(4), 297–301.
Marmot, M., & Bell, R. (2018). The sustainable development goals and health equity. Epidemiology, 29(1), 5–7.
Mendola, P., Wallace, M., Hwang, B. S., Liu, D., Robledo, C., Männistö, T., Sundaram, R., Sherman, S., Ying, Q., & Grantz, K. L. (2016). Preterm birth and air pollution: Critical windows of exposure for women with asthma. The Journal of Allergy and Clinical Immunology, 138(2), 432–440. e5.
Misiukiewicz-Stepien, P., & Paplinska-Goryca, M. (2021). Biological effect of PM10 on airway epithelium-focus on obstructive lung diseases. Clinical Immunology, 227, 108754.
Montgomery, M. T., Sajuthi, S. P., Cho, S.-H., Everman, J. L., Rios, C. L., Goldfarbmuren, K. C., Jackson, N. D., Saef, B., Cromie, M., Eng, C., Medina, V., Elhawary, J. R., Oh, S. S., Rodriguez-Santana, J., Vladar, E. K., Burchard, E. G., & Seibold, M. A. (2020). Genome-wide analysis reveals mucociliary remodeling of the nasal airway epithelium induced by urban PM2. 5. American Journal of Respiratory Cell and Molecular Biology, 63(2), 172–184.
Moser, C., & Satterthwaite, D. (2010). Toward pro-poor adaptation to climate change in the urban centers of low-and middle-income countries (pp. 231–258). Equity and vulnerability in a warming world.
Moshammer, H., Hutter, H., Hauck, H., & Neuberger, M. (2006). Low levels of air pollution induce changes of lung function in a panel of schoolchildren. The European Respiratory Journal, 27(6), 1138–1143.
Mossman, B. T., Borm, P. J., Castranova, V., Costa, D. L., Donaldson, K., & Kleeberger, S. R. (2007). Mechanisms of action of inhaled fibers, particles and nanoparticles in lung and cardiovascular diseases. Particle and Fibre Toxicology, 4(1), 1–10.
OECD I. Energy and air pollution: World energy outlook special report 2016. 2016. International Energy Agency
Olsson, D., Forsberg, B., Bråbäck, L., Geels, C., Brandt, J., Christensen, J. H., Frohn, L. M., & Oudin, A. (2021). Early childhood exposure to ambient air pollution is associated with increased risk of paediatric asthma: An administrative cohort study from Stockholm, Sweden. Environment International, 155, 106667.
Orellano, P., Quaranta, N., Reynoso, J., Balbi, B., & Vasquez, J. (2017). Effect of outdoor air pollution on asthma exacerbations in children and adults: Systematic review and multilevel meta-analysis. PLoS One, 12(3), e0174050.
O’Shaughnessy, P. J., Monteiro, A., Bhattacharya, S., & Fowler, P. A. (2011). Maternal smoking and fetal sex significantly affect metabolic enzyme expression in the human fetal liver. The Journal of Clinical Endocrinology & Metabolism, 96(9), 2851–2860.
Perez, L., Declercq, C., Iñiguez, C., Aguilera, I., Badaloni, C., Ballester, F., Bouland, C., Chanel, O., Cirarda, F. B., Forastiere, F., Forsberg, B., Haluza, D., Hedlund, B., Cambra, K., Lacasaña, M., Moshammer, H., Otorepec, P., Rodríguez-Barranco, M., Medina, S., & Künzli, N. (2013). Chronic burden of near-roadway traffic pollution in 10 European cities (APHEKOM network). The European Respiratory Journal, 42(3), 594–605.
Postma, D. S., Brightling, C., Baldi, S., Van den Berge, M., Fabbri, L. M., Gagnatelli, A., Papi, A., Van der Molen, T., Rabe, K. F., Siddiqui, S., Singh, D., Nicolini, G., Kraft, M., & ATLANTIS study group. (2019). Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): Baseline data from a prospective cohort study. The Lancet Respiratory Medicine, 7(5), 402–416.
Price, O., Walsted, E., & Hull, J. (2019). Understanding the total airway response to exercise: Current perspectives and future challenges. Current Opinion in Physiology, 10, 185–192.
Price, O. J., Ansley, L., Menzies-Gow, A., Cullinan, P., & Hull, J. H. (2013). Airway dysfunction in elite athletes–an occupational lung disease? Allergy, 68(11), 1343–1352.
Price, O. J., Sewry, N., Schwellnus, M., Backer, V., Reier-Nilsen, T., Bougault, V., Pedersen, L., Chenuel, B., Larsson, K., & Hull, J. H. (2022). Prevalence of lower airway dysfunction in athletes: A systematic review and meta-analysis by a subgroup of the IOC consensus group on ‘acute respiratory illness in the athlete’. British Journal of Sports Medicine, 56(4), 213–222.
Price, O. J., Walsted, E. S., Bonini, M., Brannan, J. D., Bougault, V., Carlsen, K. H., Couto, M., Kippelen, P., Moreira, A., Pite, H., Rukhadze, M., & Hull, J. H. (2022). Diagnosis and management of allergy and respiratory disorders in sport: An EAACI task force position paper. Allergy, 77(10), 2909–2923.
Pritchard, A., Burns, P., Correia, J., Jamieson, P., Moxon, P., Purvis, J., Thomas, M., Tighe, H., & Sylvester, K. P. (2021). ARTP statement on cardiopulmonary exercise testing 2021. BMJ Open Respiratory Research, 8(1), e001121.
Robertson, A., Dodgson, J., Collings, P., & Seaton, A. (1984). Exposure to oxides of nitrogen: Respiratory symptoms and lung function in British coalminers. Occupational and Environmental Medicine, 41(2), 214–219.
Robinson, P. D., Salimi, F., Cowie, C. T., Clifford, S., King, G. G., Thamrin, C., Hardaker, K., Mazaheri, M., Morawska, L., Toelle, B. G., & Marks, G. B. (2022). Ultrafine particle exposure and biomarkers of effect on small airways in children. Environmental Research, 214, 113860.
Robinson, R. K., Birrell, M. A., Adcock, J. J., Wortley, M. A., Dubuis, E. D., Chen, S., McGilvery, C. M., Hu, S., Shaffer, M. S. P., Bonvini, S. J., Maher, S. A., Mudway, I. S., Porter, A. E., Carlsten, C., Tetley, T. D., & Belvisi, M. G. (2018). Mechanistic link between diesel exhaust particles and respiratory reflexes. The Journal of Allergy and Clinical Immunology, 141(3), 1074–1084.
Rumchev, K., Brown, H., & Spickett, J. (2007). Volatile organic compounds: Do they present a risk to our health? Reviews on Environmental Health, 22(1), 39–56.
Ryu, M. H., Afshar, T., Li, H., Wooding, D. J., Orach, J., Zhou, J. S., Murphy, S., Lau, K. S. K., Schwartz, C., Yuen, A. C. Y., Rider, C. F., & Carlsten, C. (2022). Impact of exposure to diesel exhaust on inflammation markers and proteases in former smokers with chronic obstructive pulmonary disease: A randomized, double-blinded, crossover study. American Journal of Respiratory and Critical Care Medicine, 205(9), 1046–1052.
Sandström, T., Frew, A., Svartengren, M., & Viegi, G. (2003). The need for a focus on air pollution research in the elderly. The European Respiratory Journal, 21(40 suppl), 92s–95s.
Schikowski, T., Mills, I. C., Anderson, H. R., Cohen, A., Hansell, A., Kauffmann, F., Kramer, U., Marcon, A., Perez, L., Sunyer, J., Probst-Hensch, N., & Kunzli, N. (2014). Ambient air pollution: A cause of COPD? The European Respiratory Journal, 43(1), 250–263.
Schultz, E. S., Hallberg, J., Gustafsson, P. M., Bottai, M., Bellander, T., Bergström, A., Kull, I., Gruzieva, O., Thunqvist, P., Pershagen, G., & Melén, E. (2016). Early life exposure to traffic-related air pollution and lung function in adolescence assessed with impulse oscillometry. The Journal of Allergy and Clinical Immunology, 138(3), 930–932.
Segal, R., Martonen, T., Kim, C., & Shearer, M. (2002). Computer simulations of particle deposition in the lungs of chronic obstructive pulmonary disease patients. Inhalation Toxicology, 14(7), 705–720.
Seltzer, J., Bigby, B. G., Stulbarg, M., Holtzman, M. J., Nadel, J. A., Ueki, I. F., Leikauf, G. D., Goetzl, E. J., & Boushey, H. A. (1986). O3-induced change in bronchial reactivity to methacholine and airway inflammation in humans. Journal of Applied Physiology, 60(4), 1321–1326.
Shin, S.-W., Bae, D.-J., Park, C.-S., Lee, J.-U., Kim, R.-H., Kim, S. R., Chang, H. S., & Park, J. S. (2020). Effects of air pollution on moderate and severe asthma exacerbations. The Journal of Asthma, 57(8), 875–885.
Stewart, N. J., Smith, L. J., Chan, H.-F., Eaden, J. A., Rajaram, S., Swift, A. J., Weatherley, N. D., Biancardi, A., Collier, G. J., Hughes, D., Klafkowski, G., Johns, C. S., West, N., Ugonna, K., Bianchi, S. M., Lawson, R., Sabroe, I., Marshall, H., & Wild, J. M. (2022). Lung MRI with hyperpolarised gases: Current & future clinical perspectives. The British Journal of Radiology, 95(1132), 20210207.
Strand, V., Rak, S., Svartengren, M., & Bylin, G. (1997). Nitrogen dioxide exposure enhances asthmatic reaction to inhaled allergen in subjects with asthma. American Journal of Respiratory and Critical Care Medicine, 155(3), 881–887.
Sylvester, K. P., Clayton, N., Cliff, I., Hepple, M., Kendrick, A., Kirkby, J., Miller, M., Moore, A., Rafferty, G. F., O’Reilly, L., Shakespeare, J., Smith, L., Watts, T., Bucknall, M., & Butterfield, K. (2020). ARTP statement on pulmonary function testing 2020. BMJ open. Respiratory Research, 7(1), e000575.
Sylvester, K. P., Youngs, L., Rutter, M., Beech, R., & Mahadeva, R. (2021). Early respiratory diagnosis: Benefits of enhanced lung function assessment. BMJ Open Respiratory Research, 8(1), e001012.
Szalontai, K., Gémes, N., Furák, J., Varga, T., Neuperger, P., Balog, J. Á., Puskás, L. G., & Szebeni, G. J. (2021). Chronic obstructive pulmonary disease: Epidemiology, biomarkers, and paving the way to lung cancer. Journal of Clinical Medicine, 10(13), 2889.
Tunnicliffe, W., Burge, P., & Ayres, J. (1994). Effect of domestic concentrations of nitrogen dioxide on airway responses to inhaled allergen in asthmatic patients. The Lancet, 344(8939–8940), 1733–1736.
Wei, B., Zhou, Y., Li, Q., Zhen, S., Wu, Q., Xiao, Z., Liao, J., Zhu, B., Duan, J., Yang, X., & Liang, F. (2024). Outdoor fine particulate matter exposure and telomere length in humans: A systematic review and meta-analysis. Ecotoxicology and Environmental Safety, 275, 116206.
Weill H. Occupational lung diseases: Research approaches and methods. 2020. Routledge
WHO. Ambient (outdoor) air pollution: Key facts 2022. https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health.
Xu, Z., Li, J., & Han, Z. (2022). Numerical study of particle fouling deposition on heat transfer surface. Energy Storage and Saving, 1(1), 44–52.
Zemp, E., Elsasser, S., Schindler, C., Kunzli, N., Perruchoud, A. P., Domenighetti, G., Medici, T., Ackermann-Liebrich, U., Leuenberger, P., Monn, C., Bolognini, G., Bongard, J. P., Brändli, O., Karrer, W., Keller, R., Schöni, M. H., Tschopp, J. M., Villiger, B., & Zellweger, J. P. (1999). Long-term ambient air pollution and respiratory symptoms in adults (SAPALDIA study). American Journal of Respiratory and Critical Care Medicine, 159(4), 1257–1266.
Zhang, Y., Eckel, S. P., Berhane, K., Garcia, E., Muchmore, P., Molshatzki, N. B.-A., Rappaport, E. B., Linn, W. S., Habre, R., & Gilliland, F. D. (2021). Long-term exposures to air pollutants affect FeNO in children: A longitudinal study. The European Respiratory Journal, 58(5), 2100705.
Ziou, M., Tham, R., Wheeler, A. J., Zosky, G. R., Stephens, N., & Johnston, F. H. (2022). Outdoor particulate matter exposure and upper respiratory tract infections in children and adolescents: A systematic review and meta-analysis. Environmental Research, 210, 112969.

