Dr. Samuel K. Nyarko1, Dr. Lila M. Fernandes2, Dr. Ahmed R. El-Badry3
1 Dr. AYESHA RIAZ, Department of Medical Informatics, Imperial College London, UK
2 Dr. JONATHAN LEE, Department of Cardio-Oncology, Mayo Clinic, USA
3 Dr. MARIA SILVA, Department of Biomedical Engineering, University of São Paulo, Brazil
4 Dr. AMIR KHALID, School of Health Sciences, University of Sydney, Australia
5 Dr. LARA CHEN, Division of Oncology, National University of Singapore
6 Dr. THOMAS ABEKE, Institute of Clinical Research, University of Cape Town, South Africa
7 Dr. NINA FALK, Department of Digital Health, Karolinska Institute, Sweden
* Corresponding Author: Dr. Ayesha Riaz Email: [email protected]
Abstract
Cardiotoxicity emerging as a result of cancer therapies poses a significant risk to patient survival and post-treatment quality of life. The complexity of clinical decision-making in identifying and managing therapy-related cardiac complications presents ongoing challenges for healthcare professionals. This study explores current diagnostic and management practices, identifies clinician needs, and assesses the potential of digital health technologies to support these processes. Through semi-structured interviews with seven clinical specialists, a three-phase clinical decision model was constructed: (1) symptom recognition, (2) diagnostic evaluation with interdisciplinary consultation, and (3) therapeutic decision-making and intervention planning. The research reveals major hurdles, such as the lack of standardized diagnostic frameworks and inconsistent symptom presentation, which complicate timely diagnosis. Furthermore, clinicians highlighted the need for accurate, real-time patient-reported outcomes to support symptom tracking and early detection. These insights emphasize the necessity of integrating remote monitoring and intelligent decision-support systems into evolving clinical workflows. The paper concludes with design considerations for digital interventions aimed at enhancing clinical decision-making in cardio-oncology care.
Keywords: Collaborative decision-making, clinical decision-making, cancer treatment-induced cardiotoxicity, artificial intelligence in oncology
© The Author(s) 2025. Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third-party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
1 INTRODUCTION
Cancer remains the foremost cause of mortality globally, posing formidable challenges to public health systems worldwide [15]. While conventional cancer treatments such as chemotherapy have been extensively utilized, they frequently result in severe toxic effects on essential organs like the liver and kidneys, occasionally culminating in life-threatening complications [56, 60, 65, 76]. In response to these limitations, the past two decades have seen a paradigm shift toward the development and implementation of targeted, biologic, and immune-based therapies. Since 2010, over 180 novel drugs have gained approval [84], each aiming to enhance therapeutic efficacy by precisely targeting malignant cells while minimizing collateral damage to healthy tissue, thus improving patients’ overall quality of life [13, 42, 44, 73, 74, 85, 91]. Concurrently, continued advancements in traditional treatments, including chemotherapy, hormone therapy, and radiotherapy, have further contributed to increased survival rates and decreased immediate toxicity [33, 75, 83]. However, these advancements, coupled with extended patient survival, have surfaced new clinical challenges, notably the emergence of cancer treatment-induced cardiotoxicity, a condition previously under-recognized [33, 75, 83].
Cancer treatment-induced cardiotoxicity refers to a spectrum of cardiovascular diseases (CVDs) caused by oncologic interventions and has garnered increasing attention in clinical research [5, 10]. In specific oncologic contexts, cardiotoxicity is responsible for up to 30% of cardiovascular complications in cancer patients [2, 10, 81], significantly impacting long-term survival and quality of life [31, 34, 49, 97]. For instance, in breast cancer patients, cardiotoxicity resulting from treatment has become the primary cause of morbidity and mortality during long-term follow-up periods [40].
Despite accumulating clinical observations and evidence regarding treatment-induced cardiotoxicity, there remains a lack of standardized protocols to support systematic clinical decision-making for both the detection of cardiotoxicity and the monitoring of associated symptoms [47, 64]. In response, federal agencies have called for urgent collaborative efforts to identify at-risk populations, evaluate established and emerging strategies for CVD management, and improve clinical outcomes for cancer patients [66]. Yet, effective monitoring, accurate diagnosis, and timely intervention for cardiotoxicity-related complications continue to pose major challenges, especially in post-treatment phases when patients are no longer under direct hospital supervision [11, 47, 99]. Complicating matters further, the onset of cardiotoxicity-related CVDs can vary widely, appearing from several weeks to over a decade after cancer therapy [61]. This variability hinders the provision of consistent long-term care. Additionally, patients often lack the health literacy needed to recognize early warning signs, while clinicians face significant obstacles in implementing effective, continuous monitoring strategies outside of acute care settings [24].
To address these difficulties, a variety of patient-centered initiatives have been launched to engage cancer survivors in proactive health monitoring and risk management [23, 54, 69]. Nevertheless, a crucial gap remains: the current clinical workflows and decision-making processes used by experts to detect and monitor cardiotoxicity are not well understood. Without a nuanced understanding of the practical needs and existing barriers faced by clinicians, it becomes difficult to develop holistic strategies that prevent CVD progression and reduce associated mortality among cancer survivors.
The growing integration of digital health technologies in clinical workflows offers promising avenues to support decision-making in high-stakes scenarios such as cancer treatment-induced cardiotoxicity. Innovations including mobile health (mHealth) applications, wearable technologies, voice-assisted tools, and predictive artificial intelligence (AI) models have shown promise in enhancing cancer care and broader medical contexts [25]. For example, mHealth platforms and wearable sensors can help track patient-reported symptoms and physical activity levels, while AI-powered conversational agents have been successfully applied in public health and mental health interventions [39, 48, 71, 72, 92, 95]. Moreover, machine learning algorithms are increasingly being utilized to facilitate collaborative decision-making between human clinicians and AI systems [19, 93, 98]. However, to ensure the usability and efficacy of such technologies without introducing additional complexity or burden, the design and implementation of these tools must be grounded in an in-depth understanding of existing clinical workflows. It is essential to account for clinicians’ perspectives and concerns regarding technology adoption. In the context of managing cardiotoxicity related to cancer therapy, understanding current workflows, decision-making processes, and specific points of intervention is critical for aligning technological solutions with clinical practice. A clinician-centered approach is thus necessary to responsibly integrate digital tools into real-world medical decision-making.
To explore these issues, our study investigates the following research questions:
• RQ1: What are the current practices and needs that clinicians face in cancer treatment-induced cardiotoxicity decision-making?
• RQ2: How do clinicians envision the opportunities and concerns related to digital health technologies in the clinical management of cancer treatment-induced cardiotoxicity?
To answer these questions, we conducted semi-structured interviews with seven clinicians experienced in treating a variety of cancers using diverse therapeutic modalities. These interviews explored clinicians’ current practices and perceived needs regarding cardiotoxicity management and encouraged them to reflect on how digital tools could be integrated into their existing workflows.
Our analysis revealed a three-step structure to the current decision-making process for cardiotoxicity risk management: (1) Symptom Identification; (2) Diagnostic Testing and Specialist Collaboration; and (3) Clinical Decision-Making and Intervention. We found that clinical decision-making in this context is inherently dynamic and involves multiple stakeholders. Balancing perspectives among cardiologists, oncologists, and other specialists is often required. Clinicians emphasized the necessity for early detection and expressed strong interest in remote monitoring solutions and other digital health technologies as means to enhance their workflow.
Based on these findings, we outline several design considerations for developing future technology-supported tools that aid in collaborative clinical decision-making for managing cancer treatment-induced cardiotoxicity. We underscore the importance of appreciating the collaborative, evolving nature of decision-making in complex, uncertain clinical environments.
Our contributions are as follows:
• We identify key challenges in the diagnosis and monitoring of cancer treatment-induced cardiotoxicity from the perspective of clinical practitioners.
• We explore the potential and limitations of emerging digital technologies in supporting clinical decision-making and improving cardiotoxicity management.
• We propose design implications for the development of clinician-centered, technology-supported workflows to enhance long-term care and risk mitigation for cancer survivors.
2 REVIEW OF RELEVANT LITERATURE
2.1 Cardiotoxic Effects of Cancer Therapies
Cancer has become the foremost cause of premature mortality on a global scale [15]. Ongoing advancements in cancer treatment have significantly enhanced survival outcomes, with over 19 million individuals currently living with a history of cancer in the United States alone [2, 81]. The fundamental objective of oncologic care is to eradicate malignant cells and prevent recurrence, thereby extending patients’ lifespans [80]. This goal has spurred progress in various therapeutic modalities—including chemotherapy, targeted agents, biologic treatments, and immunotherapies—enabling remission and, in some cases, curative outcomes [16, 32]. These developments have contributed to a marked decline in cancer-related mortality over the past twenty years [43, 45, 46]. Nonetheless, the increased application of these therapies has revealed a growing concern: cardiotoxicity, which now significantly undermines the gains made in survival by introducing serious cardiovascular complications [80].
Cardiotoxicity, defined broadly as myocardial damage secondary to cancer treatment [77], has emerged as a critical determinant of both the quality of life and prognosis in cancer survivors [7, 8, 61]. It is now considered the leading cause of treatment-related morbidity and mortality among this patient population [1, 4, 9, 62]. In adults, the prevalence of cardiotoxicity may reach up to 30% depending on the specific cardiac condition [77, 96]. Initially asymptomatic, cardiotoxicity may eventually manifest through various clinical presentations such as left ventricular dysfunction, heart failure, arrhythmias, and hypertension [14, 21, 57]. Notably, evidence suggests that the risk of developing cardiotoxicity may persist and even escalate long after therapy has concluded [61].
Given its multifaceted presentation, cardiotoxicity introduces significant diagnostic and therapeutic uncertainty into cancer care. Concerns about cardiovascular toxicity may lead to the modification or discontinuation of effective treatments, potentially compromising oncologic outcomes [77, 78]. Clinicians thus face the dual challenge of delivering optimal cancer therapy while concurrently assessing and mitigating cardiac risks during both treatment planning and long-term follow-up [30, 41, 77]. Therefore, enhancing pre-treatment risk stratification and enabling early post-treatment interventions to counteract cardiotoxicity could significantly improve patient outcomes in the context of ongoing therapeutic uncertainty.
2.2 Managing Cardiotoxicity Risk:
Current Practices and Persistent Barriers
Managing cardiotoxicity risk has become increasingly critical given the widespread use of cardiotoxic cancer therapies. Approaches have primarily centered on prevention, early detection, and ongoing monitoring, and are typically delivered through patient-centered frameworks [47, 63].
Preventive measures often begin with identifying high-risk patients, closely monitoring them, and providing educational resources to improve health literacy. Despite these efforts, cancer patients frequently prioritize the urgency of cancer eradication over the management of comorbid cardiovascular conditions, such as hypertension, ischemia, or heart failure [20]. Consequently, early signs of cardiac complications may go unnoticed until more advanced and potentially irreversible cardiovascular conditions develop [12]. Timely identification of these issues is vital to ensuring early intervention, which is essential for promoting cardiac recovery [18]. Clinicians usually employ diagnostic tools such as electrocardiograms (ECGs) and echocardiograms to detect early signs of cardiotoxicity [24]. Upon suspicion, patients are typically referred to cardiologists or cardio-oncology specialists for further assessment and treatment. Continuous monitoring throughout the cancer care continuum—before, during, and after treatment—remains a critical component of effective management [51, 67, 70].
However, despite the increasing adoption of patient-centered approaches to cardiotoxicity management, considerable challenges persist. Existing clinical guidelines lack specificity regarding the evaluation and follow-up of cardiotoxicity [47]. Moreover, the evolving nature of cancer treatments and their cardiovascular implications presents further obstacles to establishing consistent, evidence-based strategies that balance oncologic efficacy with cardiovascular safety.
2.3 Emerging Digital Solutions in Cancer and Cardiotoxicity Management
The integration of digital health technologies into oncology care represents a transformative shift in how clinicians monitor and address treatment-related adverse effects. Technologies such as wearable devices (WDs), conversational agents (CAs), and artificial intelligence (AI) models are increasingly utilized to enhance patient monitoring and clinical decision-making [19, 28, 37, 92].
Wearable devices provide continuous, real-time, remote tracking of various physiological metrics [28, 37, 39]. Devices such as Fitbit and Garmin smartwatches can monitor heart rate and physical activity, while more advanced multisensory systems assess oxygen saturation, respiratory rate, sleep cycles, and circadian rhythms [28, 37, 52]. Concurrently, digital communication tools—including telehealth platforms and conversational agents—are used to collect qualitative data, such as patient-reported symptoms and treatment side effects [55]. These platforms improve access to care and promote adherence through virtual consultations and personalized coaching [22, 27, 55]. For example, the Nurse AMIE project employs smart speakers to deliver mental and informational support to women with metastatic breast cancer [72], while Gregory et al. [36] developed a mobile app to track cardiac symptoms through structured questionnaires.
Despite these technological advances, existing solutions may fall short of addressing the personalized, dynamic, and urgent nature of cardiotoxicity risk management. Additionally, barriers to adoption persist, including patient reluctance and limited integration into routine clinical practice [38, 68].
Artificial intelligence presents new opportunities to develop efficient and scalable digital health solutions for oncology. In particular, large language models (LLMs)—advanced deep learning systems capable of generating and interpreting natural language—have facilitated a range of healthcare applications, from patient communication and education to clinical decision support [29, 48, 59, 92]. LLM-powered CAs have been deployed for cancer-related symptom tracking, education, diagnosis, and decision-making support [71, 92]. Clinicians have also shown interest in AI-based predictive models that augment their decision-making, an area of growing importance in both research and practice [3, 19, 50, 53, 94]. For instance, Chang et al. [19] developed a model using clinical, chemotherapeutic, and echocardiographic variables to predict cancer treatment-related cardiac dysfunction (CTRCD), demonstrating superior accuracy compared to conventional models. Similarly, Yagi et al. [94] presented an AI model capable of robustly predicting CTRCD using baseline ECG data. Nevertheless, these models typically rely on structured clinical data and often face limitations related to reliability, transparency, and privacy concerns [3, 19, 53].
While digital health technologies offer promising avenues for remote monitoring of cancer survivors, there remains limited understanding of how these innovations can support clinicians in managing long-term cardiotoxicity risks. To address this knowledge gap, our study seeks to explore clinicians’ current workflows, challenges, and attitudes toward adopting these emerging technologies.
3 METHODS
Clinical observations in existing literature increasingly highlight the relevance of cardiotoxicity in cancer patients. Despite this, a comprehensive understanding of prevailing clinical practices in managing cardiotoxicity risks remains limited. This gap potentially impedes the formulation of clinical guidelines and the adoption of technologies to support clinical decision-making. To address this shortfall, we conducted semi-structured interviews with seven clinical experts, guided by established Human-Centered AI research design principles [6, 79, 87–90], to gain deeper insights into current practices, challenges, and needs. This study has two primary objectives:
• To obtain a nuanced understanding of clinicians’ current practices and challenges in managing cardiotoxicity risks among cancer patients.
• To investigate the potential role of advanced technologies, such as telehealth devices integrated with AI, and clinicians’ views and expectations regarding the implementation of such technologies in cardiotoxicity risk management.
3.1 Participants
We recruited seven clinicians whose roles involve the monitoring, diagnosis, and treatment of cancer patients. These specialists, from various departments and clinical backgrounds, routinely encounter cases of cancer treatment-induced cardiotoxicity in their practice. Using convenience sampling, we approached colleagues and professional contacts within relevant fields. Table 1 outlines the demographic information of the participants, including gender, department, job titles, and years of clinical experience. Each participant was assigned a unique numerical identifier (𝑃 #), which is used throughout the manuscript. Interviews were conducted remotely via Zoom and lasted approximately 40 minutes per session. Ethical approval for this research was obtained from the Institutional Review Board (IRB) at the first author’s institution.
| P# | Gender | Department | Job Title | Year of Practice |
| P1 | Female | Medical Oncology | Breast Medical Oncologist | 2 years |
| P2 | Male | Radiation Oncology | Gastrointestinal Radiation Oncologist | 6 years |
| P3 | Female | Bone and Marrow Transplant and Cellular Therapy | Hematologist | 1.5 years |
| P4 | Female | Internal Medicine | Oncologist | 13 years |
| P5 | Female | Sarcoma Center | Sarcoma Medical Oncologist | 2 years |
| P6 | Female | Thoracic and Geriatric Oncology | Thoracic and Geriatric Oncologist | 7 years |
| P7 | Male | Internal Medicine | Cardiologist | 8 years |
Table 1. Demographics of Physician Participants
3.2 Procedures
Each interview session was led by one primary researcher, with at least one additional team member present to take notes and pose clarifying questions. Informed consent was obtained from all participants prior to the interviews. The interviews were structured around two focal areas.
Initially, participants described their clinical background, area of expertise, years of practice, typical workflow, and experiences with cardiotoxicity in their clinical setting. They were then prompted to discuss a recent case involving cardiotoxicity either during or following cancer treatment. Through detailed, in-depth questioning and discussion, we explored their clinical processes, methods for managing cardiotoxicity risks, current use of technology, and notably, their perceived needs and challenges.
Following this, we sought participants’ expert opinions on the application of smart technologies in cardiotoxicity risk management. Specific follow-up questions probed their views and concerns on adopting such technologies, as well as the type of information they would find valuable in aiding clinical decision-making and improving patient outcomes.
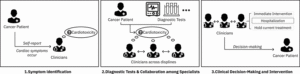
Fig. 1. Current Workflow of Cardiotoxicity Diagnosis. Initially, clinicians identify symptoms or suspect cardiotoxicity based on patient self-reports or acute cardiac symptoms. Diagnostic tests are then performed, and collaboration with other specialists takes place to confirm diagnosis. Subsequently, clinical decisions concerning immediate interventions, treatment modifications, and ongoing monitoring are made.
3.3 Data Analysis
All interviews were audio-recorded with participant consent and subsequently transcribed. Two researchers independently reviewed the transcripts to build a comprehensive understanding of the content. An open coding approach was employed [26], wherein each researcher assigned initial codes. Using an inductive process, these codes were iteratively developed into sub-themes and overarching themes. Similar codes were grouped into broader categories. To ensure reliability and validity, both researchers cross-validated the coded data, discussed discrepancies, and reached consensus through collaborative discussions. Any remaining disagreements were resolved through further deliberation with the entire research team.
4 FINDINGS
In this section, we present the findings derived from our qualitative analysis of semi-structured interviews with clinical experts. Section 4.1 outlines current clinical practices involved in the diagnosis and monitoring of cancer treatment-induced cardiotoxicity, covering the stages from symptom identification to diagnostic testing and clinical decision-making. Sections 4.2 and 4.3 then address the challenges faced in current risk management practices and explore clinicians’ perspectives on the potential role of digital health technologies in mitigating these issues and supporting clinical decisions.
4.1 Current Practices in Managing Cardiotoxicity Risk During Cancer Treatment
Following a cancer diagnosis, patients receive individualized treatment plans and follow-up schedules. Cardiotoxicity induced by cancer therapies can manifest at any stage after treatment initiation. Insights from our interviews reveal that the current management of cardiotoxicity risk involves three main phases: symptom recognition, diagnostic evaluation with interdisciplinary collaboration, and clinical decision-making with corresponding interventions, as depicted in Figure 1.
4.1.1 Symptom Recognition
The initial phase of cardiotoxicity risk management involves identifying symptoms indicative of potential cardiac complications. The complexity of this step arises from whether the cardiotoxicity presents with symptoms and whether the patient is under hospitalization.
Cancer treatment typically follows a regimen of multiple sessions or cycles over time. These plans differ significantly depending on patient-specific variables, including age, sex, medical history, cancer type, and cancer stage, thereby affecting hospital visit frequency. Generally, patients are seen at the start of each cycle, followed by treatments and assessments scheduled accordingly. For instance, P5 explained that treatment frequency varies, stating, “during chemo, it’s every 3 weeks, and then radiation is every day.”
During hospital visits, which may be for treatment or follow-up, patients undergo standard monitoring, physical exams, or therapeutic procedures. These routine measures enable clinicians to detect asymptomatic cardiotoxicity during inpatient care. As P5 recounted, a patient undergoing chemotherapy displayed bradycardia with premature ventricular contractions, identified through clinical monitoring rather than overt symptoms. Additionally, patients can self-report symptoms while hospitalized, allowing for timely assessment and intervention. However, as unanimously noted by participants, current clinical protocols primarily rely on patient self-reporting following discharge. This reliance poses a challenge for detecting asymptomatic cardiotoxicity in outpatient settings, increasing the risk of delayed diagnosis and serious complications.
Self-reported symptoms commonly associated with cardiotoxicity include chest pain, shortness of breath, fatigue, and palpitations. P2, for example, recalled a patient presenting with chest pain shortly after beginning a new treatment cycle. Patients use multiple avenues to report such symptoms, typically while at home. Many utilize MyChart, an online health portal integrated with electronic medical records (EMRs), to message their healthcare providers (P3, P4, P6). In more urgent scenarios, patients may call the clinic or emergency services directly (P5, P6). P4 described the typical patient communication patterns:
“They usually contact us. They will either write a MyChart message, or if it’s more severe than that, they will call 911, or if they just cannot wait, they’ll go to the ER.” (P4)
4.1.2 Diagnostic Evaluation and Interdisciplinary Collaboration
When symptoms are reported or identified during clinical examinations, clinicians conduct diagnostic tests—such as electrocardiograms (EKGs), echocardiograms, and lab work—to confirm cardiotoxicity. This process typically involves cross-specialty collaboration, drawing on the expertise of multiple departments to establish a definitive diagnosis. These tests and imaging modalities are critical for accurate assessment. P1, for instance, detailed the diagnostic approach taken in a complex case:
“We checked a variety of different labs… she didn’t have troponin elevation, CK elevation… no visible EKG changes. The echocardiogram looked unchanged.” (P1)
Importantly, symptoms and test findings do not always indicate cardiotoxicity; they may instead result from other medical conditions or adverse effects unrelated to cardiac toxicity. Clinicians must therefore perform a differential diagnosis to eliminate alternative explanations. P4 illustrated this approach when evaluating a fatigued patient:
“We do the full work up. We do blood counts, we do other tests. We, for example, check for their thyroid. We check for other things. If everything else is negative, then we are sure that the disease is not progressing. The fatigue is not from the disease. Then we have every reason to believe the fatigue is from the drug.” (P4)
Oncology providers frequently collaborate with specialists—most commonly cardiologists—for advanced assessments, particularly when cardiotoxicity is confirmed or symptoms are ambiguous. P1 noted that while oncologists can request advanced imaging like cardiac MRI, such decisions are often made in consultation with other relevant specialists:
“Technically, we can still order cardiac MRI. I’ll say mostly in our practice, we will consult it with either this rheumatologist or somebody in our cardinal oncology group will often be the one to support the ordering of this.” (P1)
Likewise, P5 described a typical workflow for involving cardiologists when there is evidence of cardiac dysfunction:
“Typically, if we confirm that there is a cardiac like an actual cardiac depression or event, or anything, then we get cardiologists involved. If it turns out that there is no ejection fraction problem. There’s no coronary issue or anything like that. Then typically, we’re like, okay, then it’s just the chemo(therapy), and they don’t have to see the cardiologist then. That’s at least what we typically do now.” (P5)
Given the complexity and diagnostic ambiguity of many clinical scenarios, even patients with atypical symptoms that may not obviously indicate cardiotoxicity are often referred to cardiologists to ensure a comprehensive evaluation. As P7 explained:
“It’s more than even if they have atypical complaints like atypical chest pain not really related to the heart, they tend to get referred to cardiologists.” (P7)
4.1.3 Clinical Decision-Making and Intervention
Once cardiotoxicity is suspected or confirmed, clinical decisions are made regarding immediate intervention, potential medication modifications or discontinuation, and ongoing monitoring strategies. In severe cases, hospitalization is typically the initial response. For instance, P1 described a standard protocol for managing such cases: “We admitted her to the hospital, where she then received higher doses of steroids and was considered for some of our clinical trials with cardiologists.” During the hospital stay, interventions such as cardioversion and rate control may be employed to stabilize the patient prior to discharge.
Medication adjustment or discontinuation represents another critical component of managing cardiotoxicity in cancer patients. These decisions must be carefully balanced against the patient’s broader cancer treatment objectives and the specific cardiac complications involved. Management may entail halting the suspected causative agent, modifying the therapeutic regimen, or introducing cardioprotective medications. As P4 noted, “I asked him to stop the medication right away,” underscoring the urgency often required to prevent further cardiac injury.
However, participants emphasized that discontinuation alone may not always resolve cardiotoxic symptoms. To mitigate further cardiac harm while continuing effective oncologic care, clinicians might maintain cancer therapy alongside cardiology interventions or switch to a less cardiotoxic treatment plan. Such decisions are frequently made in consultation with cardiologists, as illustrated by P7:
“Sometimes we say, like, this is clearly related to the cancer treatment. We should not continue the cancer treatment and something else needs to be done. So that’s when the cancer doctor then comes up with some other form of cancer treatment which might not be the best possible cancer treatment, but something close to best possible. But would not have the cardia(c) complication.” (P7)
Compared to patients experiencing isolated cardiac conditions, those with cancer and cardiotoxicity face greater clinical complexity and vulnerability, making treatment decisions more intricate and consequential. This dual-risk scenario can lead to highly challenging choices, particularly when the optimal cancer therapy must be altered due to cardiac side effects, as highlighted again by P7:
“…the question, then comes whether you need to continue cancer treatment or not, or whether you have to change the best possible cancer treatment that they get.” (P7)
Following the acute intervention phase, ongoing outpatient monitoring becomes critical to assess cardiac function and guide further treatment. P4 recounted an example of a patient who underwent initial hospital evaluation with normal results but was sent home with a cardiac monitor to ensure continuous surveillance: “Everything was negative, everything was normal. But they sent him home with a monitor.” For patients on revised medication regimens, regular follow-up is essential to track cardiotoxicity progression and treatment efficacy.
4.2 Challenges in the Current Clinical Practices of Cardiotoxicity Risk Management
The current methods for diagnosing and managing cardiotoxicity in cancer patients involve several significant challenges. These include difficulties in recognizing ambiguous symptoms, inconsistent diagnostic protocols, and an overreliance on patient self-reporting, all of which hinder early detection and timely intervention.
4.2.1 Challenges Related to the Diagnosis of Cardiotoxicity.
Diagnosing cardiotoxicity in cancer patients presents numerous clinical difficulties. Common challenges identified by participants include overlapping treatment-related toxicities, variability in symptom presentation, and the limitations of existing diagnostic modalities.
Ambiguity Caused by Overlapping Toxicities.
Cancer therapies frequently induce a range of adverse effects that affect multiple organ systems. Cardiotoxicity symptoms often overlap with other common chemotherapy-induced side effects, complicating the diagnostic process. For instance, as P1 noted, “if her side effect was rash. Rash is caused by those chemotherapies as well, so it is really hard to differentiate what is causing it.”
Symptoms like fatigue and shortness of breath, while frequently associated with cardiac dysfunction, are also common among patients receiving chemotherapy, making them diagnostically nonspecific. P4 described a case illustrating this challenge:
“There were more symptoms that he was having… A lot of those symptoms, fatigue, shortness of breath, you can have just from chemo(therapy)… With him specifically… the EKG was showing some changes as well… He ended up in the hospital feeling very short of breath… The ejection fraction was normal initially, but later it dropped to 30%.” (P4)
Additionally, there are cases in which a primary focus on tumor-related symptoms may lead to underappreciation of cardiac concerns. P2 emphasized this potential oversight: “Cause this was a rectal cancer patient. We don’t necessarily ask about chest pain normally because we’re more focused on like the GI part of the disease.”
Variability of Symptoms.
According to participants, the clinical manifestation of cardiotoxicity can vary considerably depending on the cancer type and treatment regimen, even with the use of similar chemotherapeutic agents. This variability complicates diagnostic clarity. Interviewees cited symptoms such as edema, fatigue, chest pain, and arrhythmias as potentially indicative of cardiotoxicity. For example, P1 described a patient undergoing immunotherapy who exhibited worsening edema and increased fatigue, while P2 reported a case of chest pain believed to stem from coronary vasospasm. This range in presentation emphasizes the complexity of cardiotoxicity diagnosis across diverse patient profiles.
Moreover, the absence of symptoms in some patients presents an additional challenge. Cardiotoxicity may remain undetected without proactive monitoring. As P4 recounted: “Because he was completely asymptomatic. He was sitting at home relaxing, and the monitor picked up this thing.”
Limitations of Existing Testing.
A further obstacle lies in the limitations of current diagnostic testing protocols. There is no universally standardized approach for identifying cancer treatment-related cardiotoxicity. Clinicians frequently rely on a combination of diagnostic tools and specialist consultations, yet these methods often yield inconclusive findings. P1 highlighted this issue:
“We checked a variety of different labs… She had liver enzyme increases… But she didn’t have troponin elevation. CK elevation. Some of these things that we more often see with cardiotoxicity. She had no visible EKG changes… The echocardiogram looked unchanged.” (P1)
Participants also expressed concern about the sensitivity of standard laboratory tests in detecting early or subtle cardiac damage. P1 remarked, “something that we weren’t finding based on those labs, because those labs are, you know, somewhat limited sometimes in diagnosing some of these cardiac changes.”
Interpreting diagnostic results can also be problematic. P5 cited a patient whose cardiac lab values and imaging results did not reveal clear abnormalities despite the presence of symptoms.
Timeliness emerged as another critical limitation. Delays in conducting essential tests—such as echocardiograms or EKGs—can significantly postpone diagnosis and therapeutic intervention. As P2 stated, “I had to wait. I had to wait to get the echo. I had to wait to get the stress echo. I had to wait to get the EKG, so basically, everything was kind of delayed.”
4.2.2 Challenges Related to the Monitoring of Cardiotoxicity
Beyond the diagnostic difficulties, monitoring cardiotoxicity during or following cancer therapy presents its own set of significant challenges.
Timing and Methods of Monitoring
The unpredictability of cardiotoxicity onset complicates decisions regarding both the timing and modality of monitoring. Cardiotoxic effects may emerge at any stage—during active treatment or well after its completion—regardless of whether patients are hospitalized or recovering at home. As P5 stated, “a lot of times they’ll develop cardiotoxicity 6 months later, sometimes even a year or 2 later.” Some treatment regimens have more defined temporal risk profiles. For instance, P5 noted that patients who do not return to baseline health following the expected recovery period from chemotherapy—typically 7 to 10 days—may be exhibiting signs of more severe complications, including cardiotoxicity. Prolonged regimens, such as immunotherapy or inhibitor-based treatments, necessitate ongoing surveillance over extended durations. As P1 emphasized, due to the unpredictable emergence of side effects, continuous vigilance is required throughout the treatment course:
“these side effects can happen at any time during their treatment. I think certain side effects are more likely to happen earlier in the course versus later. This patient had already received almost 3 months of therapy… the problem is that these patients sometimes need to be on immunotherapy for a year… So you kind of have to be on the lookout at all times for these issues.” (P1)
Cardiotoxicity monitoring predominantly occurs in clinical settings during scheduled follow-up appointments. However, the frequency and scope of these follow-ups vary based on factors such as cancer type, treatment modality, symptom presentation, and patient health status. This variability complicates monitoring, particularly for patients receiving outpatient care. P1 highlighted logistical issues some patients face, including difficulty accessing timely follow-up due to geographic distance, which can lead to delayed recognition and management of cardiotoxic effects.
Furthermore, monitoring guidelines are not uniform across treatment types. As P7 described, certain cancer therapies are associated with established echocardiographic surveillance protocols, while others lack such recommendations:
“So the cancer treatments which typically cause the heart function to get worse, there [are] guidelines or their cancer [has] guidelines, where they sometimes recommend getting an echo(cardiograms). but it’s not there, and some cancer treatments which do not affect the heart function at all.” (P7)
Limitations of Self-Reported Symptom Monitoring
Although in-hospital monitoring allows for timely detection and management of adverse cardiac events, outpatient care relies heavily on patients self-reporting symptoms—a process fraught with challenges. One of the most frequently cited issues is underreporting or minimization of symptoms, even when patients are encouraged to be forthcoming. As P1 remarked, “There are a lot of patients that they are hesitant to call us in with symptoms.. I think some patients will downplay what’s actually going on.”
Participants provided nuanced explanations for this trend. A common reason was patients’ reluctance to seem burdensome or overly dramatic about their condition. As P1 explained, “I think it’s also they don’t want to feel like they’re complaining too much.” Another crucial factor is limited health literacy and fear of treatment disruption. Patients often worry that reporting symptoms might result in dose reductions or even discontinuation of therapy, thus potentially compromising treatment efficacy. P1 illustrated this concern:
“they’re concerned about efficacy not being as good, or that I’m gonna stop their treatment because of toxicity”
In addition, the burden of managing multiple symptomatic medications discourages some patients from reporting new or worsening symptoms. The use of drugs to manage side effects such as nausea or diarrhea can be overwhelming and disruptive to daily routines, leading patients to tolerate symptoms rather than add further medications.
Delayed responses from healthcare providers further discourage patients from reaching out. In resource-limited settings, even when patients do report symptoms, timely medical attention is often unavailable. Past experiences of delayed care may lead to resignation and reluctance to report new concerns. Geographic and logistical barriers further compound the issue, especially in underserved areas where patients must travel long distances to access care. As P1 observed:
“A lot of our patients, especially here, they travel very long distances to come here for treatment. Oftentimes, they may call and be having an event but can’t get in to see us the same day or even within the week… They don’t have the resources, even if they did have changes in their symptoms, to get out here or be treated.” (P1)
Additionally, existing tools for self-reporting are not tailored specifically to capture cardiotoxicity symptoms. As P5 pointed out, “We will use a survey tool called Promise 10 to ask patients how they’re feeling. But it’s not specific to cardiotoxicity.”
Failure to report symptoms in a timely manner can result in critical delays in diagnostic testing and therapeutic intervention. P2 noted the consequences of such delays:
“you know she had the symptoms. Then she told me, like, essentially 4 or 5 days later. and then I had to wait. I had to wait to get the echo. I had to wait to get the stress echo. I had to wait to get the Kkg, so basically, everything was kind of delayed.”
4.2.3 Lack of Clinical Guidelines
Many of the aforementioned challenges are compounded by the absence of standardized clinical guidelines for the identification and surveillance of cardiotoxicity resulting from cancer treatment. This deficit is especially apparent in relation to symptom recognition and diagnostic evaluation.
P2 acknowledged that during standard oncology assessments, cardiac considerations are often not prioritized:
“In oncology clinics, we do a general review of systems, but there aren’t a lot of specifics about cardiac issues” (P2)
This gap may be attributed to the rapid evolution of oncologic therapies and their associated toxicities, which tends to outpace the development of evidence-based clinical protocols. P1 highlighted this lag in formal guidance:
“So much of this that it’s very new, and the guidelines for how we diagnose and identify it probably lag the actual clinical, you know, outcome and what we see in the clinic.” (P1)
The absence of well-established guidelines leads to inconsistencies in monitoring and care. P7 emphasized this disparity, noting that while some patients receive rigorous cardiac evaluations, others may not, depending on the presence or absence of treatment-specific recommendations:
“so the cancer treatments which typically cause the heart function to get worse, there are guidelines or their cancer has guidelines, where they sometimes recommend getting echocardiograms. But it’s not there for some cancer treatments which do not affect heart function at all.”
4.3 Clinicians’ Needs & Perspectives Towards Potential Technology-Supported Solutions
In light of the diagnostic and monitoring challenges associated with cardiotoxicity, clinicians shared their perspectives and identified key needs where digital health technologies, particularly AI-driven tools, could offer support.
Early Detection and Intervention.
A central need articulated by participants is the capacity for early identification and diagnosis of cardiotoxicity. Early symptom detection significantly improves the likelihood of preventing severe adverse events. P1 emphasized that prompt recognition of side effects is particularly critical due to the overlapping toxicities of chemotherapeutic agents:
“Sometimes they’re very mild, like people can have a little bit of diarrhea. If it’s small, then that’s something that will pass. But if it’s a true colitis that the patient is having, that could be life-threatening. So determining what those are earlier on helps.” (P1)
However, the ability to detect cardiotoxicity early is often constrained by the availability of specialized resources and expertise. This challenge is more pronounced in generalist or resource-limited settings, potentially delaying accurate diagnosis and intervention. P1 elaborated:
“It’s also very helpful for them (cancer specialists) to know when these patients need to be referred to somebody here or in a different center where they are evaluated by a cardio-oncology specialist… those patients have worse outcomes because we know delay in treatment with these cardiac events can be serious.” (P1)
4.3.1 Existing Supportive Tools.
Participants cited several existing tools that provide some degree of clinical support.
One such tool is the Chemotherapy Risk Assessment Scale for High-age Patients (CRASH), which estimates the likelihood of severe hematologic and non-hematologic toxicities in older adults undergoing chemotherapy. While CRASH can contribute to early detection, clinicians noted it requires manual computation, adding to their workload. P6 highlighted that automating tools like CRASH could significantly improve clinical efficiency.
Another tool mentioned is the electronic Frailty Index (eFI), an automated measure based on electronic health record (EHR) data used to assess frailty in older populations. Although integrated into some clinical systems, eFI lacks specificity for cardiotoxicity risk, limiting its utility in this context.
Participants also referenced AI technologies such as Deep 6, designed to match patient cancer profiles to clinical trials. While potentially useful for reducing the time and effort involved in patient recruitment, this tool has seen limited implementation in routine clinical workflows.
Overall, while current tools offer partial support, their limitations—especially in automation and cardiotoxicity-specific utility—highlight the need for more targeted technological solutions.
4.3.2 Potentials for Remote Monitoring.
Participants reflected on the growing role of telehealth and wearable devices in enabling remote symptom monitoring and early detection.
Currently, remote monitoring technologies are typically deployed for patients already identified as having cardiac risk. Devices like temporary heart monitors are frequently used to detect irregular heart rhythms, especially in patients presenting symptoms such as palpitations (P4, P5, P7). Clinicians expressed optimism that telehealth could enable continuous data collection between clinic visits, particularly benefiting patients who face barriers to frequent in-person care. P1 described the challenge of capturing episodic symptoms during appointments:
“I’ve had a couple of patients that are on potentially cardiotoxic agents, and they’ll have episodes of almost passing out. But I’m not sure if, like, that was correlated to something else. So, and obviously they’re not often having them in the office when I’m seeing them, so that could be helpful.” (P1)
Clinicians also noted that such technologies could empower patients to engage more proactively in their care. P1 mentioned that some cancer patients are highly invested in their treatment journey and are open to using tools that provide insight into their health. Wearable devices like smartwatches may enhance patient awareness and support informed decision-making.
4.3.3 Perspectives Towards AI Technologies.
Given the challenges with manual processes, clinicians were asked to reflect on the potential role of advanced AI technologies, such as chatbots and predictive risk scoring systems.
Although clinicians are aware of the emerging role of AI in EHR integration, its practical adoption in clinical environments remains limited. P4 illustrated this sentiment:
“I don’t know why I don’t use any right now. I haven’t felt the need to use any at this point.” (P4)
Overall, clinicians conveyed that AI should act as a supplementary resource rather than a replacement for clinical judgment. P5 emphasized:
“I know one thing that the physicians would definitely want to just make sure—that it’s an adjunct versus the only decision maker.” (P5)
P4 echoed this:
“AI is supposed to be my assistant, not my replacement.” (P4)
Furthermore, clinicians emphasized the need for AI tools to provide clear and actionable insights. P7 noted that an influx of raw data could overwhelm clinicians unless paired with meaningful, usable outputs.
Several participants (P1, P2, P4, P7) recognized the potential for AI to streamline documentation and reduce administrative burdens. As P4 observed:
“There’s a lot of burnout in medicine, and the burnout is from all of this documentation that we do. And I see a huge role of AI in that documentation that will help with the burnout.” (P4)
Regarding predictive risk tools, participants supported the automation of risk calculations. P6 emphasized the efficiency that such automation could bring:
“It would be nice if it could be done automatically.” (P6)
4.3.4 Concerns About Digital Health Solutions.
Despite recognizing the benefits of AI and telehealth, clinicians also voiced critical concerns, particularly around accuracy, data overload, alert fatigue, ethical considerations, and workflow integration.
A primary issue raised involves understanding and validating AI-generated risk scores. Clinicians stressed the importance of interpretability and evidence-based validation. P3 commented:
“You need to validate and say, well, a risk score from one to 4, a very low risk for a heart attack.” (P3)
In addition, clinicians questioned how these scores could be practically integrated into decision-making processes. Several participants (P1, P5, P6, P7) voiced similar concerns:
“What do we do with it?”
This underscores the demand for AI outputs to come with clear guidance on interpretation and next steps.
Alarm fatigue and data overload emerged as further challenges. Excessive alerts from remote monitoring devices and digital systems may desensitize providers to critical warnings. P4 noted:
“But you know, doctors also get annoyed about being sent every little detail. We don’t want to be woken up by every little thing, so it should not be annoying like that.” (P4)
The volume of incoming data, unless filtered and structured appropriately, may also add to the clinician’s workload rather than alleviate it.
Ethical concerns also featured prominently in clinicians’ feedback. One major issue involved responsibility for monitoring new streams of patient data. Clinicians questioned who would bear responsibility for continuously tracking and responding to this information. P1 explained:
“There’s always the issue of ethics… if something like that happens like, when do we find out about this? Is it ethical like for us to wait to see this data every time they come in? Versus? How are we gonna have somebody monitoring this data all the time, like the next man call, who’s gonna be monitoring this.” (P1)
This concern is closely tied to institutional capacity and infrastructure—ensuring there are adequate resources to manage and respond to alerts in a timely manner.
Finally, liability in relation to AI-driven recommendations also raised apprehension. Clinicians discussed who would be accountable if patients were advised by AI systems to seek emergency care. P5 questioned:
“Do we have the chatbot recommend an intervention such as going to the ER, or is it more so that the chatbot then gets flagged that contact the nurse or doctor now?” (P5)
5 DISCUSSION
Diagnosing and monitoring cardiotoxicity in oncology patients remains a significant concern due to the symptom complexity, individual variability, and life-threatening consequences involved. This clinical context exemplifies a high-stakes, uncertain, and constantly evolving scenario. Section 4 explored the existing challenges in clinical practice, examined technological applications, and reviewed concerns expressed by clinicians. In this section, we interpret our findings in relation to existing literature and outline design implications for future research and technological innovation to enhance clinicians’ workflows. Importantly, we examine the deeper challenges embedded within dynamic clinical environments and the dominant collaborative practices shaping clinical expert workflows.
5.1 Design Considerations
A key finding from Section 4.3 relates to clinicians’ prioritisation of early cardiotoxicity detection. Participants underscored that delayed diagnosis can lead to serious health risks. Building on their insights regarding technology’s potential in improving early detection and cardiotoxicity risk management, we propose several design considerations addressing technological functionality, integration with clinical research, and accessibility.
Potential of Remote Monitoring Technologies: Continuous Surveillance
Remote monitoring solutions offer promising support for continuous patient care post-discharge. Although patients receive systematic monitoring during hospital stays, the risk of complications may escalate once they return home. Prior research has demonstrated that telehealth systems and wearable technologies can enable real-time health monitoring [28, 37, 39]. Our participants recognized the utility of remote technologies in maintaining continuous observation and encouraging patient self-management. Prior studies have implemented wearables to monitor parameters such as heart rate and oxygen saturation in oncology patients, proving effective in delivering real-time clinical data to support ongoing care [28, 37, 52].
Potential of AI Technologies: Predictive Analytics for Risk
AI-driven tools exhibit substantial promise in cardiotoxicity risk prediction, a critical aspect of early detection. For example, recent evidence [93] shows that AI models trained on clinical, chemotherapeutic, and echocardiographic data can accurately predict cardiac dysfunction related to cancer therapy. Such predictive capabilities allow for proactive management strategies and earlier clinical intervention, potentially mitigating the severity of cardiotoxic effects.
Potential of AI Technologies: Automating Clinical Documentation
AI can also enhance efficiency through automation of routine documentation tasks, aiding early detection by reallocating clinician time. Participants noted that many administrative tasks demand substantial manual input. Automating repetitive, low-risk tasks—such as data entry and preliminary risk assessments—can improve accuracy, streamline workflows, and reduce human error. This would allow clinicians to focus on complex patient interactions and nuanced decision-making.
Despite the promising capabilities of these technologies, we propose the following considerations for their design and implementation to ensure clinical utility and patient safety:
Functionality and Responsibility Distribution
Participants emphasized the importance of carefully designing remote monitoring tools in terms of functionality and role allocation. For instance, a chatbot monitoring daily symptoms must be equipped to understand symptom variability and determine appropriate follow-up actions. A key question involves whether such a system should offer recommendations when patients report concerning symptoms and how it should respond during emergencies. These functions must clearly be framed as supportive advice rather than definitive medical decisions to avoid misunderstandings or liability issues. Furthermore, responsibility for interpreting the continuous data stream generated by these technologies must be clearly assigned, as mishandling such data could have serious consequences.
Sociotechnical Dimensions
Deploying remote monitoring in outpatient care must address sociotechnical challenges, including differences in patients’ health and digital literacy, economic barriers, and accessibility limitations. These factors influence technology adoption and usability. Designers must consider these disparities to ensure technologies are inclusive, intuitive, and usable by a broad patient population.
Purpose and Integration of Technologies
Participants highlighted the necessity of clearly defining the function and intended use of new technologies. These tools should complement—not replace—clinical judgment, providing decision support and actionable insights without increasing the burden on clinicians. Seamless integration with existing workflows is essential for adoption and sustainability.
Clinical Research Alignment
To be effective, remote monitoring and AI tools must be informed by clinical evidence and aligned with current research. Identification of key monitoring parameters and cardiotoxicity indicators is crucial. Collaboration among clinicians, patients, and HCI researchers is necessary to establish clinically valid alert thresholds and safety criteria for these technologies.
5.2 The Importance of Collaborative Paradigms in Clinical Expert Workflows
Throughout our interviews, the prominence of collaborative models in managing cancer-related cardiotoxicity was consistently emphasized. Oncologists frequently consult with other medical specialists, especially cardiologists, to formulate diagnostic and treatment strategies. Such interprofessional collaboration is characteristic of clinical scenarios where complex comorbidities demand nuanced expertise across domains. While essential for delivering holistic patient care, this collaborative practice introduces notable challenges.
Among the most critical of these are the timing and structure of referrals and the methods through which inter-specialist collaboration is conducted. Determining when to initiate a referral is crucial, as both premature and delayed referrals may adversely affect patient outcomes. Furthermore, whether a referral entails a singular consultation or an ongoing partnership significantly influences treatment decisions. The mechanisms of collaboration—including the communication channels and tools employed for information exchange—also play a pivotal role.
To illustrate challenges related to collaborative modalities, consider a scenario in which a patient undergoing cancer therapy begins exhibiting early cardiotoxic symptoms, detected by a cardiologist. In response, the cardiologist might recommend suspending the cancer treatment to mitigate cardiac risks. However, from an oncological standpoint, such an adjustment may compromise the effectiveness of cancer therapy. Although all involved specialists share the goal of optimizing patient care, their divergent clinical priorities may lead to contradictory recommendations. Resolving these differences requires the coordinated exchange of clinical knowledge, negotiation, and occasionally, compromise to achieve a unified treatment strategy that prioritizes the patient’s overall well-being.
Furthermore, patients are integral to this collaborative ecosystem. Delivering patient-centered care necessitates incorporating the patient’s values, concerns, and preferences into the decision-making process. Active patient participation helps mitigate power imbalances often present in clinical consultations, ensuring that their perspectives are not overlooked. Effective communication with patients about the risks and benefits of treatment options should extend beyond information delivery—it should be empathetic, respectful, and grounded in an understanding of the patient’s lived experiences.
The efficacy and timeliness of communication between specialists and patients are fundamental to collaborative clinical decisions. We advocate for future HCI research to explore when collaborative decision-making is most needed, the priorities and values of each clinician involved, and the processes through which they communicate and collaborate in practice. Moreover, any design and evaluation of technology intended to support clinical decision-making should be rooted in actual collaborative workflows. These tools must be tested within real-world clinical environments, ensuring they are capable of addressing the multifaceted dynamics of collaboration while fostering meaningful patient engagement.
5.3 The Evolving Nature of Clinical Settings
Our study highlights the dynamic and rapidly transforming landscape of cancer therapies and related clinical practices for cardiotoxicity diagnosis and monitoring. This continuous evolution is critical for researchers to consider, as novel therapies are regularly introduced, validated, and adopted, necessitating frequent adjustments to clinical routines.
The emergence of new cancer treatments requires healthcare professionals to quickly assimilate unfamiliar workflows and toxicity profiles. Cardiotoxicity detection and monitoring workflows are especially fluid due to their intersection with multiple specialties, compelling clinicians to integrate diverse perspectives in making informed treatment decisions. For instance, chemotherapy protocols have undergone significant evolution over time. Earlier regimens may have focused predominantly on gastrointestinal complications; however, newer therapies that extend patient survival have brought previously rare issues, such as cardiotoxicity, to the forefront.
Looking ahead, future therapies with largely uncharacterized toxicity profiles may uncover previously undocumented symptoms or adverse effects, necessitating vigilant clinical observation and continuous research. While traditional chemotherapeutic agents have posed notable risks to hepatic and renal function [56, 60, 65, 76], emerging treatments appear to shift toxicity toward cardiovascular outcomes—thus altering the primary causes of treatment-related mortality. As new therapies emerge, attention may shift to yet unidentified complications, requiring clinicians to remain adaptable in both monitoring and management practices.
This evolving treatment landscape frequently results in a lag between clinical advances and the development of corresponding guidelines, tools, and surveillance strategies. This delay is particularly evident in cardiotoxicity management for cancer patients, where a lack of up-to-date, specific guidelines presents a significant challenge [47, 77]. Our participants echoed this concern, noting that the pace of guideline development often fails to keep up with therapeutic innovations. Clinicians, therefore, play a vital dual role as both decision-makers and frontline observers. Their experiential insights are invaluable in guiding HCI researchers, clinical investigators, and policy developers toward recognizing emerging issues and designing appropriate interventions.
The continuous transformation of treatment modalities and associated adverse effects necessitates adaptive decision-making processes and evolving workflows, including changes in monitoring durations, diagnostic protocols, and biomarker usage. To effectively support clinical decision-making amid such variability, HCI researchers must proactively track shifts in clinical practice and ensure that their technological solutions align with the evolving needs of clinical care.
5.4 Limitations and Directions for Future Research
This study presents several limitations. First, the participant pool was relatively small, comprising seven physicians—a sample size consistent with expert-focused studies in related literature [17, 86, 98]. Future investigations should aim to involve a broader, more diverse cohort across multiple clinical institutions. Despite this limitation, our adherence to rigorous interview research methodologies [35] enabled us to reach thematic saturation, offering a robust understanding of current physician practices, the challenges they encounter, and their emerging expectations for future technological support.
Secondly, the heterogeneity of cardiotoxicity—arising from varying cancer types, treatments, and individual patient profiles—remains a significant obstacle. For practical implementation of deployable systems, initial efforts should focus on narrowly defined patient populations, such as individuals with a single cancer type undergoing standardized treatment protocols. Such targeted research will facilitate the development of precise, adaptable solutions that can subsequently be expanded to accommodate broader populations.
Moreover, although our emphasis on oncologists and cardiologists was essential to investigate the interplay between cancer therapies and cardiotoxicity, we recognize the need for a more interdisciplinary perspective. Including emergency physicians, nurse practitioners, and other frontline healthcare professionals would provide a more comprehensive view of the clinical landscape. Additionally, involving patients in the research process is critical. Understanding their lived experiences and preferences is vital to designing inclusive and effective technological tools.
6 CONCLUSION
This study provides important insights into the pressing challenges and opportunities associated with the management of cancer treatment-induced cardiotoxicity. Through in-depth interviews with clinical experts, we elucidated the complex and layered decision-making processes involved in cardiotoxicity risk assessment, including symptom identification, diagnostic testing, and therapeutic interventions. Our findings reveal notable clinical challenges, such as symptom variability, overlapping toxicities, and the lack of unified clinical protocols, all of which complicate accurate and timely diagnoses. Additional barriers in symptom monitoring—such as underreporting by patients and difficulties in maintaining long-term follow-up—further underscore the need for novel, technology-driven solutions.
Our exploration of digital health technologies suggests that clinicians recognize the potential benefits of tools designed for early detection, remote monitoring, and intelligent decision support. However, the development and deployment of these tools must prioritize seamless integration into established clinical workflows while addressing clinician concerns around usability, efficiency, and reliability.
We offer several design recommendations for future technological interventions, with a focus on clinician-centered approaches. Key considerations include enhancing early detection capabilities, enabling reliable symptom tracking, and fostering collaborative decision-making. This work contributes to a deeper understanding of how digital health technologies can be thoughtfully developed and implemented to support risk management of cardiotoxicity in cancer care, ultimately leading to improved outcomes for patients.
References
- Husam Abdel-Qadir, Peter C Austin, Douglas S Lee, Eitan Amir, Jack V Tu, Paaladinesh Thavendiranathan, Kinwah Fung, and Geoffrey M Anderson. 2017. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA cardiology 2, 1 (2017), 88–93.
- Daniel Addison, Tomas G Neilan, Ana Barac, Marielle Scherrer-Crosbie, Tochi M Okwuosa, Juan C Plana, Kerryn W Reding, Viviany R Taqueti, Eric H Yang, Vlad G Zaha, et al. 2023. Cardiovascular imaging in contemporary cardio- oncology: a scientific statement from the American Heart Association. Circulation 148, 16 (2023), 1271–1286.
- Bouatmane Ahmed, Daaif Abdelaziz, and Bousselham Abdelmajid. 2024. Advancements in Cardiotoxicity Detection and Assessment through Artificial Intelligence: A Comprehensive Review. In 2024 4th International Conference on Innovative Research in Applied Science, Engineering and Technology (IRASET). 1–8. https://doi.org/10.1109/IRASET60544.
- Joachim Alexandre, Jennifer Cautela, Stéphane Ederhy, Ghandi Laurent Damaj, Joe-Elie Salem, Fabrice Barlesi, Laure Farnault, Aude Charbonnier, Mariana Mirabel, Stéphane Champiat, et al. 2020. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European cardio-oncology guidelines. Journal of the American Heart Association 9, 18 (2020), e018403.
- Raza M Alvi, Matthew J Frigault, Michael G Fradley, Michael D Jain, Syed S Mahmood, Magid Awadalla, Dae Hyun Lee, Daniel A Zlotoff, Lili Zhang, Zsofia D Drobni, et al. 2019. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T). Journal of the American College of Cardiology 74, 25 (2019), 3099–3108.
- Saleema Amershi, Dan Weld, Mihaela Vorvoreanu, Adam Fourney, Besmira Nushi, Penny Collisson, Jina Suh, Shamsi Iqbal, Paul N Bennett, Kori Inkpen, et al. 2019. Guidelines for human-AI interaction. In Proceedings of the 2019 chi conference on human factors in computing systems. 1–13.
- Saro H Armenian, Gregory T Armstrong, Gregory Aune, Eric J Chow, Matthew J Ehrhardt, Bonnie Ky, Javid Moslehi, Daniel A Mulrooney, Paul C Nathan, Thomas D Ryan, et al. 2018. Cardiovascular disease in survivors of childhood cancer: insights into epidemiology, pathophysiology, and prevention. Journal of clinical oncology 36, 21 (2018), 2135–2144.
- Saro H Armenian, Christina Lacchetti, Ana Barac, Joseph Carver, Louis S Constine, Neelima Denduluri, Susan Dent, Pamela S Douglas, Jean-Bernard Durand, Michael Ewer, et al. 2017. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology 35, 8 (2017), 893–911.
- Gregory T Armstrong, Yan Chen, Yutaka Yasui, Wendy Leisenring, Todd M Gibson, Ann C Mertens, Marilyn Stovall, Kevin C Oeffinger, Smita Bhatia, Kevin R Krull, et al. 2016. Reduction in late mortality among 5-year survivors of childhood cancer. New England Journal of Medicine 374, 9 (2016), 833–842.
- Florian Baptiste, Jennifer Cautela, Yan Ancedy, Noémie Resseguier, Thérèse Aurran, Laure Farnault, Marion Escudier, Chloé Ammar, Mélanie Gaubert, Charles Dolladille, et al. 2019. High incidence of atrial fibrillation in patients treated with ibrutinib. Open Heart 6, 1 (2019), e001049.
- Ana Barac, Gillian Murtagh, Joseph R Carver, Ming Hui Chen, Andrew M Freeman, Joerg Herrmann, Cezar Iliescu, Bonnie Ky, Erica L Mayer, Tochi M Okwuosa, et al. 2015. Cardiovascular health of patients with cancer and cancer survivors: a roadmap to the next level. Journal of the American College of Cardiology 65, 25 (2015), 2739–2746.
- Janice M Bonsu, Avirup Guha, Lawrence Charles, Vedat O Yildiz, Lai Wei, Brandee Baker, Jonathan E Brammer, Farrukh Awan, Maryam Lustberg, Raquel Reinbolt, et al. 2020. Reporting of cardiovascular events in clinical trials supporting FDA approval of contemporary cancer therapies. Journal of the American College of Cardiology 75, 6 (2020), 620–628.
- Hossein Borghaei, Luis Paz-Ares, Leora Horn, David R Spigel, Martin Steins, Neal E Ready, Laura Q Chow, Everett E Vokes, Enriqueta Felip, Esther Holgado, et al. 2015. Nivolumab versus docetaxel in advanced nonsquamous non–small- cell lung cancer. New England Journal of Medicine 373, 17 (2015), 1627–1639.
- Erin J Aiello Bowles, Robert Wellman, Heather Spencer Feigelson, Adedayo A Onitilo, Andrew N Freedman, Thomas Delate, Larry A Allen, Larissa Nekhlyudov, Katrina AB Goddard, Robert L Davis, et al. 2012. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. Journal of the National Cancer Institute 104, 17 (2012), 1293–1305.
- Freddie Bray, Mathieu Laversanne, Elisabete Weiderpass, and Isabelle Soerjomataram. 2021. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 127, 16 (2021), 3029–3030.
- Howard Broder, Roberta A Gottlieb, and Norman E Lepor. 2008. Chemotherapy and cardiotoxicity. Reviews in cardiovascular medicine 9, 2 (2008), 75.
- Carrie J Cai, Samantha Winter, David Steiner, Lauren Wilcox, and Michael Terry. 2019. ” Hello AI”: uncovering the onboarding needs of medical practitioners for human-AI collaborative decision-making. Proceedings of the ACM on Human-computer Interaction 3, CSCW (2019), 1–24.
- Daniela Cardinale, Alessandro Colombo, Giuseppina Lamantia, Nicola Colombo, Maurizio Civelli, Gaia De Giacomi, Mara Rubino, Fabrizio Veglia, Cesare Fiorentini, and Carlo M Cipolla. 2010. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. Journal of the American College of Cardiology 55, 3 (2010), 213–220.
- Wei-Ting Chang, Chung-Feng Liu, Yin-Hsun Feng, Chia-Te Liao, Jhi-Joung Wang, Zhih-Cherng Chen, Hsiang-Chun Lee, and Jhih-Yuan Shih. 2022. An Artificial Intelligence Approach for Predicting Cardiotoxicity in Breast Cancer Patients Receiving Anthracycline. Archives of Toxicology 96, 10 (Oct. 2022), 2731–2737. https://doi.org/10.1007/s00204-022-03341-y
- Carol L Chen. 2015. Cardiovascular prevention in the cancer survivor. Current atherosclerosis reports 17 (2015), 1–10.
- Jersey Chen, Jessica B Long, Arti Hurria, Cynthia Owusu, Richard M Steingart, and Cary P Gross. 2012. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. Journal of the American College of Cardiology 60, 24 (2012), 2504–2512.
- Yan-Ya Chen, Bing-Sheng Guan, Ze-Kai Li, and Xing-Yi Li. 2018. Effect of telehealth intervention on breast cancer patients’ quality of life and psychological outcomes: a meta-analysis. Journal of Telemedicine and Telecare 24, 3 (2018), 157–167.
- Feixiong Cheng and Joseph Loscalzo. 2017. Autoimmune cardiotoxicity of cancer immunotherapy. Trends in immunology
38, 2 (2017), 77–78.
- Robyn A Clark, Tania S Marin, Alexandra L McCarthy, Julie Bradley, Suchi Grover, Robyn Peters, Christos S Karapetis, John J Atherton, and Bogda Koczwara. 2019. Cardiotoxicity after cancer treatment: a process map of the patient treatment journey. Cardio-Oncology 5 (2019), 1–11.
- Steven B Clauser, Edward H Wagner, Erin J Aiello Bowles, Leah Tuzzio, and Sarah M Greene. 2011. Improving modern cancer care through information technology. American journal of preventive medicine 40, 5 (2011), S198–S207.
- Juliet Corbin and Anselm Strauss. 2014. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Sage publications.
- Anna Cox, Grace Lucas, Afrodita Marcu, Marianne Piano, Wendy Grosvenor, Freda Mold, Roma Maguire, and Emma Ream. 2017. Cancer survivors’ experience with telehealth: a systematic review and thematic synthesis. Journal of medical Internet research 19, 1 (2017), e11.
- Suzanne M Cox, Ashley Lane, and Samuel L Volchenboum. 2018. Use of wearable, mobile, and sensor technology in cancer clinical trials. JCO clinical cancer informatics 2 (2018), 1–11.
- Andrea Cuadra, Justine Breuch, Samantha Estrada, David Ihim, Isabelle Hung, Derek Askaryar, Marwan Hassanien, Kristen L. Fessele, and James A. Landay. 2024. Digital Forms for All: A Holistic Multimodal Large Language Model Agent for Health Data Entry. ACM Interact. Mob. Wearable Ubiquitous Technol. 8, 2, Article 72 (may 2024), 39 pages. https://doi.org/10.1145/3659624
- Giuseppe Curigliano, Daniela Cardinale, Susan Dent, Carmen Criscitiello, Olexiy Aseyev, Daniel Lenihan, and Carlo Maria Cipolla. 2016. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA: a cancer journal for clinicians 66, 4 (2016), 309–325.
- Iyad N Daher, Tina R Daigle, Nirmanmoh Bhatia, and Jean-Bernard Durand. 2012. The prevention of cardiovascular disease in cancer survivors. Texas Heart Institute Journal 39, 2 (2012), 190.
- Susan Dent, Peter Liu, Christine Brezden-Masley, and Daniel Lenihan. 2015. Cancer and cardiovascular disease: the complex labyrinth. Journal of Oncology 2015 (2015).
- Maria Florescu, Mircea Cinteza, and Dragos Vinereanu. 2013. Chemotherapy-induced cardiotoxicity. Maedica 8, 1 (2013), 59.
- Chunkit Fung, Sophie D Fossa, Michael T Milano, Deepak M Sahasrabudhe, Derick R Peterson, and Lois B Travis. 2015. Cardiovascular disease mortality after chemotherapy or surgery for testicular nonseminoma: a population-based study. Journal of Clinical Oncology 33, 28 (2015), 3105–3115.
- Patricia I Fusch Ph D and Lawrence R Ness. 2015. Are we there yet? Data saturation in qualitative research. (2015).
- Megan E Gregory, Weidan Cao, Saurabh Rahurkar, Pallavi Jonnalagadda, James C Stock, Sanam M Ghazi, Endia Reid, Abigail L Berk, Courtney Hebert, Lang Li, et al. 2023. Exploring the Incorporation of a Novel Cardiotoxicity Mobile Health App Into Care of Patients With Cancer: Qualitative Study of Patient and Provider Perspectives. JMIR cancer 9, 1 (2023), e46481.
- Gillian Gresham, Andrew E Hendifar, Brennan Spiegel, Elad Neeman, Richard Tuli, BJ Rimel, Robert A Figlin, Curtis L Meinert, Steven Piantadosi, and Arvind M Shinde. 2018. Wearable activity monitors to assess performance status and predict clinical outcomes in advanced cancer patients. NPJ digital medicine 1, 1 (2018), 27.
- Sarah J Hardcastle, Maddison Galliott, Brigid M Lynch, Nga H Nguyen, Paul A Cohen, Ganendra Raj Mohan, Niloufer J Johansen, and Christobel Saunders. 2018. Acceptability and utility of, and preference for wearable activity trackers amongst non-metropolitan cancer survivors. PLoS One 13, 12 (2018), e0210039.
- Sarah J Hardcastle, Ruth Jiménez-Castuera, Chloe Maxwell-Smith, Max K Bulsara, and Dana Hince. 2020. Fitbit wear-time and patterns of activity in cancer survivors throughout a physical activity intervention and follow-up: Exploratory analysis from a randomised controlled trial. PloS one 15, 10 (2020), e0240967.
- Mariana L Henry, Jiangong Niu, Ning Zhang, Sharon H Giordano, and Mariana Chavez-MacGregor. 2018. Cardiotoxicity and cardiac monitoring among chemotherapy-treated breast cancer patients. JACC: Cardiovascular Imaging 11, 8 (2018), 1084–1093.
- Joerg Herrmann. 2020. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nature Reviews Cardiology 17, 8 (2020), 474–502.
- F Stephen Hodi, Steven J O’day, David F McDermott, Robert W Weber, Jeffrey A Sosman, John B Haanen, Rene Gonzalez, Caroline Robert, Dirk Schadendorf, Jessica C Hassel, et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine 363, 8 (2010), 711–723.
- Nadia Howlader, Lynn AG Ries, Angela B Mariotto, Marsha E Reichman, Jennifer Ruhl, and Kathleen A Cronin. 2010. Improved estimates of cancer-specific survival rates from population-based data. JNCI: Journal of the National Cancer Institute 102, 20 (2010), 1584–1598.
- Yongxian Hu, Mingming Zhang, Tingting Yang, Zhuomao Mo, Guoqing Wei, Ruirui Jing, Houli Zhao, Rongrong Chen, Cheng Zu, Tianning Gu, et al. 2024. Sequential CD7 CAR T-cell therapy and allogeneic HSCT without GVHD New England Journal of Medicine 390, 16 (2024), 1467–1480.
- Ahmedin Jemal, Elizabeth Ward, Yongping Hao, and Michael Thun. 2005. Trends in the leading causes of death in the United States, 1970-2002. Jama 294, 10 (2005), 1255–1259.
- Ahmedin Jemal, Elizabeth Ward, and Michael Thun. 2010. Declining death rates reflect progress against cancer. PloS one 5, 3 (2010), e9584.
- Ruxandra Jurcut, Hans Wildiers, Javier Ganame, Jan D’hooge, Robert Paridaens, and Jens-Uwe Voigt. 2008. Detection and monitoring of cardiotoxicity—what does modern cardiology offer? Supportive Care in Cancer 16 (2008), 437–445.
- Taewan Kim, Seolyeong Bae, Hyun Ah Kim, Su-Woo Lee, Hwajung Hong, Chanmo Yang, and Young-Ho Kim. 2024. MindfulDiary: Harnessing Large Language Model to Support Psychiatric Patients’ Journaling. In Proceedings of the CHI Conference on Human Factors in Computing Systems (Honolulu, HI, USA) (CHI ’24). Association for Computing Machinery, New York, NY, USA, Article 701, 20 pages. https://doi.org/10.1145/3613904.3642937
- Christos Kourek, Maria Touloupaki, Athanasios Rempakos, Konstantinos Loritis, Elias Tsougkos, Ioannis Paraskevaidis, and Alexandros Briasoulis. 2022. Cardioprotective strategies from cardiotoxicity in cancer patients: a comprehensive Journal of Cardiovascular Development and Disease 9, 8 (2022), 259.
- Jennifer M. Kwan, Evangelos K. Oikonomou, Mariana L. Henry, and Albert J. Sinusas. 2022. Multimodality Ad- vanced Cardiovascular and Molecular Imaging for Early Detection and Monitoring of Cancer Therapy-Associated Cardiotoxicity and the Role of Artificial Intelligence and Big Data. Frontiers in Cardiovascular Medicine 9 (March 2022). https://doi.org/10.3389/fcvm.2022.829553
- Bonnie Ky, Mary Putt, Heloisa Sawaya, Benjamin French, James L Januzzi, Igal A Sebag, Juan Carlos Plana, Victor Cohen, Jose Banchs, Joseph R Carver, et al. 2014. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. Journal of the American College of Cardiology 63, 8 (2014), 809–816.
- Walter Lachenmeier and Dirk W Lachenmeier. 2022. Home monitoring of oxygen saturation using a low-cost wearable device with haptic feedback to improve sleep quality in a lung cancer patient: A case report. Geriatrics 7, 2 (2022), 43.
- Jessica Castrillon Lal and Feixiong Cheng. 2023. Artificial Intelligence for Risk Assessment of Cancer Therapy-Related Cardiotoxicity and Precision Cardio-Oncology. In Machine Learning and Deep Learning in Computational Toxicology,
Huixiao Hong (Ed.). Springer International Publishing, Cham, 563–578. https://doi.org/10.1007/978-3-031-20730-3_24
- Carolyn M Larsen and Sharon L Mulvagh. 2017. Cardio-oncology: what you need to know now for clinical practice and echocardiography. Echo Research & Practice 4, 1 (2017), R33–R41.
- Jamie L Larson, Adam B Rosen, and Fernando A Wilson. 2018. The effect of telehealth interventions on quality of life of cancer patients: a systematic review and meta-analysis. Telemedicine and e-Health 24, 6 (2018), 397–405.
- Joannie Lefebvre and Ilya G Glezerman. 2017. Kidney toxicities associated with novel cancer therapies. Advances in Chronic Kidney Disease 24, 4 (2017), 233–240.
- Carrie G Lenneman and Douglas B Sawyer. 2016. Cardio-oncology: an update on cardiotoxicity of cancer-related Circulation research 118, 6 (2016), 1008–1020.
- Steven E Lipshultz, M Jacob Adams, Steven D Colan, Louis S Constine, Eugene H Herman, Daphne T Hsu, Melissa M Hudson, Leontien C Kremer, David C Landy, Tracie L Miller, et al. 2013. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation 128, 17 (2013), 1927–1995.
- Zilin Ma, Yiyang Mei, Yinru Long, Zhaoyuan Su, and Krzysztof Z. Gajos. 2024. Evaluating the Experience of LGBTQ+ People Using Large Language Model Based Chatbots for Mental Health Support. In Proceedings of the CHI Conference on Human Factors in Computing Systems (Honolulu, HI, USA) (CHI ’24). Association for Computing Machinery, New York, NY, USA, Article 872, 15 pages. https://doi.org/10.1145/3613904.3642482
- Jolanta Malyszko, Petra Tesarova, Giovambattista Capasso, and Anna Capasso. 2020. The link between kidney disease and cancer: complications and treatment. The Lancet 396, 10246 (2020), 277–287.
- Lillian R Meacham, Eric J Chow, Kirsten K Ness, Kala Y Kamdar, Yan Chen, Yutaka Yasui, Kevin C Oeffinger, Charles A Sklar, Leslie L Robison, and Ann C Mertens. 2010. Cardiovascular risk factors in adult survivors of pediatric cancer—a report from the childhood cancer survivor study. Cancer epidemiology, biomarkers & prevention 19, 1 (2010), 170–181.
- Ann C Mertens, Yutaka Yasui, Joseph P Neglia, John D Potter, Mark E Nesbit Jr, Kathy Ruccione, W Anthony Smithson, and Leslie L Robison. 2001. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. Journal of Clinical Oncology 19, 13 (2001), 3163–3172.
- Pratik Mondal, Diwakar Jain, Wilbert S Aronow, and William H Frishman. 2019. Cardiotoxicity of cancer therapies. Cardiology in Review 27, 5 (2019), 230–235.
- Javid J Moslehi, Joe-Elie Salem, Jeffrey A Sosman, Bénédicte Lebrun-Vignes, and Douglas B Johnson. 2018. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. The Lancet 391, 10124 (2018), 933.
- Pablo Munoz-Schuffenegger, Sylvia Ng, and Laura A Dawson. 2017. Radiation-induced liver toxicity. In Seminars in radiation oncology, Vol. 27. Elsevier, 350–357.
- National Institutes of Health (NIH). 2021. NOT-CA-22-001: Notice of Special Interest (NOSI): Administrative Supple- ments for Research on Women’s Health in the IDeA States. https://grants.nih.gov/grants/guide/notice-files/NOT-CA- 22-001.html Accessed: 2024-07-02.
- Kazuaki Negishi, Tomoko Negishi, James L Hare, Brian A Haluska, Juan Carlos Plana, and Thomas H Marwick. 2013. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. Journal of the American Society of Echocardiography 26, 5 (2013), 493–498.
- Timothy K Nguyen, Glenn S Bauman, Christopher J Watling, and Karin Hahn. 2017. Patient-and family-centered care: a qualitative exploration of oncologist perspectives. Supportive Care in Cancer 25 (2017), 213–219.
- Allison Padegimas, Suparna Clasen, and Bonnie Ky. 2020. Cardioprotective strategies to prevent breast cancer therapy-induced cardiotoxicity. Trends in cardiovascular medicine 30, 1 (2020), 22–28.
- Noemi Pavo, Markus Raderer, Martin Hülsmann, Stephanie Neuhold, Christopher Adlbrecht, Guido Strunk, Georg Goliasch, Heinz Gisslinger, Günther G Steger, Michael Hejna, et al. 2015. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart 101, 23 (2015), 1874–1880.
- Antoine Piau, Rachel Crissey, Delphine Brechemier, Laurent Balardy, and Fati Nourhashemi. 2019. A smartphone Chatbot application to optimize monitoring of older patients with cancer. International journal of medical informatics 128 (2019), 18–23.
- Ling Qiu, Bethany Kanski, Shawna Doerksen, Renate Winkels, Kathryn H Schmitz, and Saeed Abdullah. 2021. Nurse AMIE: Using Smart Speakers to Provide Supportive Care Intervention for Women with Metastatic Breast Cancer. In Extended Abstracts of the 2021 CHI Conference on Human Factors in Computing Systems (Yokohama, Japan) (CHI EA ’21). Association for Computing Machinery, New York, NY, USA, Article 302, 7 pages. https://doi.org/10.1145/3411763. 3451827
- Eric Raymond, Laetitia Dahan, Jean-Luc Raoul, Yung-Jue Bang, Ivan Borbath, Catherine Lombard-Bohas, Juan Valle, Peter Metrakos, Denis Smith, Aaron Vinik, et al. 2011. Sunitinib malate for the treatment of pancreatic neuroendocrine New England Journal of Medicine 364, 6 (2011), 501–513.
- Peter Schmid, Sylvia Adams, Hope S Rugo, Andreas Schneeweiss, Carlos H Barrios, Hiroji Iwata, Véronique Diéras, Roberto Hegg, Seock-Ah Im, Gail Shaw Wright, et al. 2018. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. New England Journal of Medicine 379, 22 (2018), 2108–2121.
- Amir Y Shaikh and Jeffrey A Shih. 2012. Chemotherapy-induced cardiotoxicity. Current heart failure reports 9 (2012), 117–127.
- Carl Shanholtz. 2001. Acute life-threatening toxicity of cancer treatment. Critical care clinics 17, 3 (2001), 483–502.
- Nonniekaye Shelburne, Bishow Adhikari, Joanna Brell, Myrtle Davis, Patrice Desvigne-Nickens, Andrew Freedman, Lori Minasian, Thomas Force, and Scot C Remick. 2014. Cancer treatment–related cardiotoxicity: current state of knowledge and future research priorities. Journal of the National Cancer Institute 106, 9 (2014), dju232.
- Nonniekaye Shelburne, Naoko I Simonds, Bishow Adhikari, Michael Alley, Patrice Desvigne-Nickens, Eileen Dimond, Kelly Filipski, Lisa Gallicchio, and Lori Minasian. 2019. Changing hearts and minds: improving outcomes in cancer treatment-related cardiotoxicity. Current Oncology Reports 21 (2019), 1–9.
- Ben Shneiderman. 2022. Human-centered AI. Oxford University Press.
- Rebecca Siegel, Carol DeSantis, Katherine Virgo, Kevin Stein, Angela Mariotto, Tenbroeck Smith, Dexter Cooper, Ted Gansler, Catherine Lerro, Stacey Fedewa, et al. 2012. Cancer treatment and survivorship statistics, 2012. CA: a cancer journal for clinicians 62, 4 (2012), 220–241.
- Rebecca L Siegel, Kimberly D Miller, Hannah E Fuchs, and Ahmedin Jemal. 2022. Cancer statistics, 2022. CA: a cancer journal for clinicians 72, 1 (2022).
- Shreya Tadas, Jane Dickson, and David Coyle. 2023. Using Patient-Generated Data to Support Cardiac Rehabilitation and the Transition to Self-Care. In Proceedings of the 2023 CHI Conference on Human Factors in Computing Systems (Hamburg, Germany) (CHI ’23). Association for Computing Machinery, New York, NY, USA, Article 329, 16 pages. https://doi.org/10.1145/3544548.3580822
- Judy Truong, Andrew T Yan, Gemma Cramarossa, and Kelvin KW Chan. 2014. Chemotherapy-induced cardiotoxicity: detection, prevention, and management. Canadian Journal of Cardiology 30, 8 (2014), 869–878.
- S. Food and Drug Administration. [n. d.]. Drugs@FDA. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed: 2024-06-28.
- Inge M van der Sluis, Paola de Lorenzo, Rishi S Kotecha, Andishe Attarbaschi, Gabriele Escherich, Karsten Nysom, Jan Stary, Alina Ferster, Benoit Brethon, Franco Locatelli, et al. 2023. Blinatumomab added to chemotherapy in infant lymphoblastic leukemia. New England Journal of Medicine 388, 17 (2023), 1572–1581.
- Himanshu Verma, Jakub Mlynar, Roger Schaer, Julien Reichenbach, Mario Jreige, John Prior, Florian Evéquoz, and Adrien Depeursinge. 2023. Rethinking the role of AI with physicians in oncology: revealing perspectives from clinical and research workflows. In Proceedings of the 2023 CHI Conference on Human Factors in Computing Systems. 1–19.
- Dakuo Wang, Elizabeth Churchill, Pattie Maes, Xiangmin Fan, Ben Shneiderman, Yuanchun Shi, and Qianying Wang. 2020. From human-human collaboration to Human-AI collaboration: Designing AI systems that can work together with people. In Extended abstracts of the 2020 CHI conference on human factors in computing systems. 1–6.
- Dakuo Wang, Liuping Wang, Zhan Zhang, Ding Wang, Haiyi Zhu, Yvonne Gao, Xiangmin Fan, and Feng Tian. 2021. “Brilliant AI doctor” in rural clinics: Challenges in AI-powered clinical decision support system deployment. In Proceedings of the 2021 CHI conference on human factors in computing systems. 1–18.
- Dakuo Wang, Justin D Weisz, Michael Muller, Parikshit Ram, Werner Geyer, Casey Dugan, Yla Tausczik, Horst Samulowitz, and Alexander Gray. 2019. Human-AI collaboration in data science: Exploring data scientists’ perceptions of automated AI. Proceedings of the ACM on human-computer interaction 3, CSCW (2019), 1–24.
- Liuping Wang, Dakuo Wang, Feng Tian, Zhenhui Peng, Xiangmin Fan, Zhan Zhang, Mo Yu, Xiaojuan Ma, and Hongan Wang. 2021. Cass: Towards building a social-support chatbot for online health community. Proceedings of the ACM on Human-Computer Interaction 5, CSCW1 (2021), 1–31.
- Michael L Wang, Simon Rule, Peter Martin, Andre Goy, Rebecca Auer, Brad S Kahl, Wojciech Jurczak, Ranjana H Advani, Jorge E Romaguera, Michael E Williams, et al. 2013. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. New England Journal of Medicine 369, 6 (2013), 507–516.
- Lu Xu, Leslie Sanders, Kay Li, James CL Chow, et al. 2021. Chatbot for health care and oncology applications using artificial intelligence and machine learning: systematic review. JMIR cancer 7, 4 (2021), e27850.
- Ryuichiro Yagi, Shinichi Goto, Yukihiro Himeno, Yoshinori Katsumata, Masahiro Hashimoto, Calum A MacRae, and Rahul C Deo. 2024. Artificial intelligence-enabled prediction of chemotherapy-induced cardiotoxicity from baseline Nature communications 15, 1 (2024), 2536.
- Ryuichiro Yagi, Shinichi Goto, Yukihiro Himeno, Yoshinori Katsumata, Masahiro Hashimoto, Calum A. MacRae, and Rahul C. Deo. 2024. Artificial Intelligence-Enabled Prediction of Chemotherapy-Induced Cardiotoxicity from Baseline Nature Communications 15, 1 (March 2024), 2536. https://doi.org/10.1038/s41467-024-45733-x
- Ziqi Yang, Xuhai Xu, Bingsheng Yao, Ethan Rogers, Shao Zhang, Stephen Intille, Nawar Shara, Guodong Gordon Gao, and Dakuo Wang. 2024. Talk2Care: An LLM-based Voice Assistant for Communication between Healthcare Providers and Older Adults. ACM Interact. Mob. Wearable Ubiquitous Technol. 8, 2, Article 73 (may 2024), 35 pages. https://doi.org/10.1145/3659625
- Edward TH Yeh and Courtney L Bickford. 2009. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. Journal of the American College of Cardiology 53, 24 (2009), 2231–2247.
- Paul Youn, Michael T Milano, Louis S Constine, and Lois B Travis. 2014. Long-term cause-specific mortality in survivors of adolescent and young adult bone and soft tissue sarcoma: a population-based study of 28,844 patients. Cancer 120, 15 (2014), 2334–2342.
- Shao Zhang, Jianing Yu, Xuhai Xu, Changchang Yin, Yuxuan Lu, Bingsheng Yao, Melanie Tory, Lace M. Padilla, Jeffrey Caterino, Ping Zhang, and Dakuo Wang. 2024. Rethinking Human-AI Collaboration in Complex Medical Decision Making: A Case Study in Sepsis Diagnosis. In Proceedings of the CHI Conference on Human Factors in Computing Systems (Honolulu, HI, USA) (CHI ’24). Association for Computing Machinery, New York, NY, USA, Article 445, 18 pages. https://doi.org/10.1145/3613904.3642343
- Ping-Pin Zheng, Jin Li, and Johan M Kros. 2018. Breakthroughs in modern cancer therapy and elusive cardiotoxicity: Critical research-practice gaps, challenges, and insights. Medicinal research reviews 38, 1 (2018), 325–376.

