
2.6
Impact Factor
Q1
ranking
The Global Journal of Social Development Studies (JSDS) focuses on three main areas of discussion: community empowerment, corporate social responsibility, and social policy. The journal welcomes papers that discuss the following themes: Social Movement and Empowerment; Human Rights, Citizenship and Development; Social Entrepreneurship; Community Development Theories, Approaches and Methods; Community Organization and Participation; Socio-cultural, Environmental and Economic Development; Industrial Relations; Decent Work; Education and Social Policy; Health Insurance and Policy; Social Protection; Wellbeing, Welfare, and Development; Poverty and Social Justice; Social Inclusion; Gender, Family and Development; Digital Technology and Development; Welfare Regime.
Nurhadi
Department of Social Development and Welfare, Universitas Gadjah Mada, Indonesia
Janianton Damanik
Department of Social Development and Welfare, Universitas Gadjah Mada, Indonesia
Suzanna Eddyono
Department of Social Development and Welfare, Universitas Gadjah Mada, Indonesia
Sari Viciawati Machdum
Department of Social Welfare, Universitas Indonesia
Kafa Abdallah Kafaa
Department of Social Development and Welfare, Universitas Gadjah Mada, Indonesia
Tauchid Komara Yuda
Department of Social Development and Welfare, Universitas Gadjah Mada, Indonesia
Pinurba Parama Pratiyudha
Department of Social Development and Welfare, Universitas Gadjah Mada, Indonesia
Saqib Fardan Ahmada
Department of Social Development and Welfare, Universitas Gadjah Mada, Indonesia
Wahid Nur Kartiko
Department of Social Development and Welfare, Universitas Gadjah Mada, Indonesia
Amellya Putri Kaharu
Department of Social Development and Welfare, Universitas Gadjah Mada, Indonesia
Hempri Suyatna
Department of Social Development and Welfare, Universitas Gadjah Mada, Indonesia
Bahruddin
Department of Social Development and Welfare, Universitas Gadjah Mada, Indonesia
Milda Longgeita Pinem
Department of Social Development and Welfare, Universitas Gadjah Mada, Indonesia
Bambang Shergi Laksmono
Department of Social Welfare, Universitas Indonesia, Indonesia
Binahayati Rusyidi
Department of Social Welfare, Universitas Padjadjaran, Indonesia
Isbandi Rukminto Adi
Department of Social Welfare, Universitas Indonesia, Indonesia
Johanna Debora Imelda
Department of Social Welfare, Universitas Indonesia, Indonesia
Medhy Aginta Hidayat
Department of Sociology, Universitas Trunojoyo Madura, Indonesia
Novi Kurnia
Department of Communication Science, Universitas Gadjah Mada, Indonesia
Amalinda Savirani
Department of Politics and Government, Universitas Gadjah Mada, Indonesia
Suharko
Department of Sociology, Universitas Gadjah Mada, Indonesia
M. Falikul Isbah
Department of Sociology, Universitas Gadjah Mada, Indonesia
Pajar Hatma Indra Jaya
Department of Islamic Community Development, Universitas Islam Nasional Sunan Kalijaga, Indonesia
Carol Chan
Facultad de Ciencias Sociales, Universidad Academia de Humanismo Cristiano, Chile
Economics
Authors wishing to include figures, tables, or text passages that have already been published elsewhere are required to obtain permission from the copyright owner(s) for both the print and online format and to include evidence that such permission has been granted when submitting their papers. Any material received without such evidence will be assumed to originate from the authors.
Please follow the hyperlink
Submit manuscript
and upload all of your manuscript files following the instructions given on the screen for
Please ensure you provide all relevant editable source files at every submission and revision. Failing to submit a complete set of editable source files will result in your article not being considered for review. For your manuscript text please always submit in common word processing formats such as .docx or LaTeX.
Please ensure to choose one sub-discipline category which is most suitable for your article from the drop-down list in the Editorial Manager.
Note:It is mandatory to suggest two or more reviewers (Name, Affiliation, Field of Expertise and Email id) at the time of submission.
Conflicts may be financial, academic, commercial, political or personal. Financial interests may include employment, research funding (received or pending), stock or share ownership, patents, payment for lectures or travel, consultancies, nonfinancial support, or any fiduciary interest in a company.
Authors must declare all such interests (or their absence) in writing upon submission of a manuscript. This conflict declaration includes conflicts or potential conflicts of all listed authors. If any conflicts are declared, the journal will publish them with the paper. In cases of doubt, the circumstance should be disclosed so that the editors may assess its significance.
The statement shall be written in a separate section before the Acknowledgments.
Authors are expected to provide a short description of the contributions made by each listed author. This too will be published in a separate section after the Conflict of Interest statement.
Manuscripts should be submitted in Word.
Headings
Please use no more than three levels of displayed headings.
Abbreviations
Abbreviations should be defined at first mention and used consistently thereafter.
Footnotes
Footnotes can be used to give additional information, which may include the citation of a reference included in the reference list. They should not consist solely of a reference citation, and they should never include the bibliographic details of a reference. They should also not contain any figures or tables.
Footnotes to the text are numbered consecutively; those to tables should be indicated by superscript lower-case letters (or asterisks for significance values and other statistical data). Footnotes to the title or the authors of the article are not given reference symbols.
Always use footnotes instead of endnotes.
Acknowledgments
Acknowledgments of people, grants, funds, etc. should be placed in a separate section on the title page. The names of funding organizations should be written in full.
Scientific style
Citation
Reference citations in the text should be identified by numbers in square brackets. Some examples:
The list of references should only include works that are cited in the text and that have been published or accepted for publication. Personal communications and unpublished works should only be mentioned in the text.
The entries in the list should be numbered consecutively.
If available, please always include DOIs as full DOI links in your reference list (e.g. “https://doi.org/abc”).
If you are unsure, please use the full journal title.
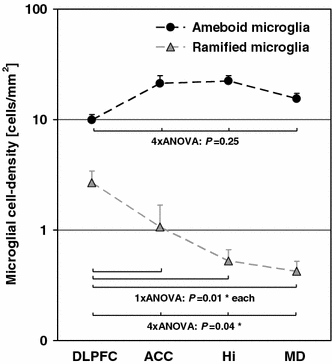
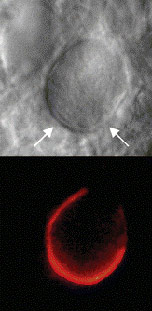
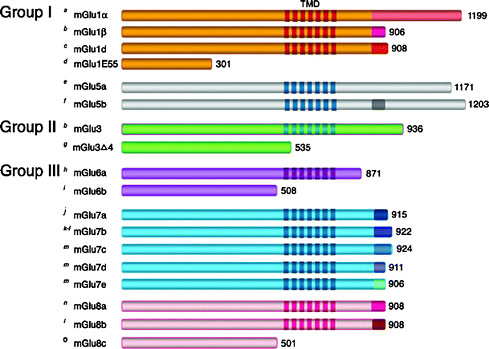
To ensure objectivity and transparency in research and to ensure that accepted principles of ethical and professional conduct have been followed, authors should include information regarding sources of funding, potential conflicts of interest (financial or non-financial), informed consent if the research involved human participants, and a statement on welfare of animals if the research involved animals.
Authors should include the following statements (if applicable) in a separate section entitled “Compliance with Ethical Standards” when submitting a paper:
Please note that standards could vary slightly per journal dependent on their peer review policies (i.e. single or double blind peer review) as well as per journal subject discipline. Before submitting your article check the instructions following this section carefully.
The corresponding author should be prepared to collect documentation of compliance with ethical standards and send if requested during peer review or after publication.
The Editors reserve the right to reject manuscripts that do not comply with the above-mentioned guidelines. The author will be held responsible for false statements or failure to fulfill the above-mentioned guidelines.
Authors are requested to disclose interests that are directly or indirectly related to the work submitted for publication. Interests within the last 3 years of beginning the work (conducting the research and preparing the work for submission) should be reported. Interests outside the 3-year time frame must be disclosed if they could reasonably be perceived as influencing the submitted work. Disclosure of interests provides a complete and transparent process and helps readers form their own judgments of potential bias. This is not meant to imply that a financial relationship with an organization that sponsored the research or compensation received for consultancy work is inappropriate.
Editorial Board Members and Editors are required to declare any competing interests and may be excluded from the peer review process if a competing interest exists. In addition, they should exclude themselves from handling manuscripts in cases where there is a competing interest. This may include – but is not limited to – having previously published with one or more of the authors, and sharing the same institution as one or more of the authors. Where an Editor or Editorial Board Member is on the author list we recommend they declare this in the competing interests section on the submitted manuscript. If they are an author or have any other competing interest regarding a specific manuscript, another Editor or member of the Editorial Board will be assigned to assume responsibility for overseeing peer review. These submissions are subject to the exact same review process as any other manuscript. Editorial Board Members are welcome to submit papers to the journal. These submissions are not given any priority over other manuscripts, and Editorial Board Member status has no bearing on editorial consideration.
Interests that should be considered and disclosed but are not limited to the following:
Funding: Research grants from funding agencies (please give the research funder and the grant number) and/or research support (including salaries, equipment, supplies, reimbursement for attending symposia, and other expenses) by organizations that may gain or lose financially through publication of this manuscript.
Employment: Recent (while engaged in the research project), present or anticipated employment by any organization that may gain or lose financially through publication of this manuscript. This includes multiple affiliations (if applicable).
Financial interests: Stocks or shares in companies (including holdings of spouse and/or children) that may gain or lose financially through publication of this manuscript; consultation fees or other forms of remuneration from organizations that may gain or lose financially; patents or patent applications whose value may be affected by publication of this manuscript.
It is difficult to specify a threshold at which a financial interest becomes significant, any such figure is necessarily arbitrary, so one possible practical guideline is the following: “Any undeclared financial interest that could embarrass the author were it to become publicly known after the work was published.”
Non-financial interests: In addition, authors are requested to disclose interests that go beyond financial interests that could impart bias on the work submitted for publication such as professional interests, personal relationships or personal beliefs (amongst others). Examples include, but are not limited to: position on editorial board, advisory board or board of directors or other type of management relationships; writing and/or consulting for educational purposes; expert witness; mentoring relations; and so forth.
Primary research articles require a disclosure statement. Review articles present an expert synthesis of evidence and may be treated as an authoritative work on a subject. Review articles therefore require a disclosure statement. Other article types such as editorials, book reviews, comments (amongst others) may, dependent on their content, require a disclosure statement. If you are unclear whether your article type requires a disclosure statement, please contact the Editor-in-Chief.
Please note that, in addition to the above requirements, funding information (given that funding is a potential competing interest (as mentioned above)) needs to be disclosed upon submission of the manuscript in the peer review system. This information will automatically be added to the Record of CrossMark, however it is not added to the manuscript itself. Under ‘summary of requirements’ (see below) funding information should be included in the ‘Declarations’ section.
Summary of requirements
The above should be summarized in a statement and placed in a ‘Declarations’ section before the reference list under a heading of ‘Funding’ and/or ‘Competing interests’. Other declarations include Ethics approval, Consent, Data, Material and/or Code availability and Authors’ contribution statements.
Please see the various examples of wording below and revise/customize the sample statements according to your own needs.
When all authors have the same (or no) conflicts and/or funding it is sufficient to use one blanket statement.
Examples of statements to be used when funding has been received:
Examples of statements to be used when there is no funding:
Examples of statements to be used when there are interests to declare:
Non-financial interests: Author C is an unpaid member of committee Z.
Non-financial interests: Author A is on the board of directors of Y and receives no compensation as member of the board of directors.
Non-financial interests: none.
Non-financial interests: Author D has served on advisory boards for Company M, Company N and Company O.
Examples of statements to be used when authors have nothing to declare:
Authors are responsible for correctness of the statements provided in the manuscript. See also Authorship Principles. The Editor-in-Chief reserves the right to reject submissions that do not meet the guidelines described in this section.
Ethics approval
When reporting a study that involved human participants, their data or biological material, authors should include a statement that confirms that the study was approved (or granted exemption) by the appropriate institutional and/or national research ethics committee (including the name of the ethics committee) and certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. If doubt exists whether the research was conducted in accordance with the 1964 Helsinki Declaration or comparable standards, the authors must explain the reasons for their approach, and demonstrate that an independent ethics committee or institutional review board explicitly approved the doubtful aspects of the study. If a study was granted exemption from requiring ethics approval, this should also be detailed in the manuscript (including the reasons for the exemption).
Retrospective ethics approval
If a study has not been granted ethics committee approval prior to commencing, retrospective ethics approval usually cannot be obtained and it may not be possible to consider the manuscript for peer review. The decision on whether to proceed to peer review in such cases is at the Editor’s discretion.
Ethics approval for retrospective studies
Although retrospective studies are conducted on already available data or biological material (for which formal consent may not be needed or is difficult to obtain) ethics approval may be required dependent on the law and the national ethical guidelines of a country. Authors should check with their institution to make sure they are complying with the specific requirements of their country.
Ethics approval for case studies
Case reports require ethics approval. Most institutions will have specific policies on this subject. Authors should check with their institution to make sure they are complying with the specific requirements of their institution and seek ethics approval where needed. Authors should be aware to secure informed consent from the individual (or parent or guardian if the participant is a minor or incapable)
Cell lines
If human cells are used, authors must declare in the manuscript: what cell lines were used by describing the source of the cell line, including when and from where it was obtained, whether the cell line has recently been authenticated and by what method. If cells were bought from a life science company the following need to be given in the manuscript: name of company (that provided the cells), cell type, number of cell line, and batch of cells.
It is recommended that authors check the NCBI database for misidentification and contamination of human cell lines. This step will alert authors to possible problems with the cell line and may save considerable time and effort.
Further information is available from the International Cell Line Authentication Committee (ICLAC).
Authors should include a statement that confirms that an institutional or independent ethics committee (including the name of the ethics committee) approved the study and that informed consent was obtained from the donor or next of kin.
Research Resource Identifiers (RRID)
Research Resource Identifiers (RRID) are persistent unique identifiers (effectively similar to a DOI) for research resources. This journal encourages authors to adopt RRIDs when reporting key biological resources (antibodies, cell lines, model organisms and tools) in their manuscripts.
Examples:
Organism: Filip1tm1a(KOMP)Wtsi RRID:MMRRC_055641-UCD
Cell Line: RST307 cell line RRID:CVCL_C321
Antibody: Luciferase antibody DSHB Cat# LUC-3, RRID:AB_2722109
Plasmid: mRuby3 plasmid RRID:Addgene_104005
Software: ImageJ Version 1.2.4 RRID:SCR_003070
Clinical Trial Registration
The World Health Organization (WHO) definition of a clinical trial is “any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes”. The WHO defines health interventions as “A health intervention is an act performed for, with or on behalf of a person or population whose purpose is to assess, improve, maintain, promote or modify health, functioning or health conditions” and a health-related outcome is generally defined as a change in the health of a person or population as a result of an intervention.
To ensure the integrity of the reporting of patient-centered trials, authors must register prospective clinical trials (phase II to IV trials) in suitable publicly available repositories. For example www.clinicaltrials.gov or any of the primary registries that participate in the WHO International Clinical Trials Registry Platform.
The trial registration number (TRN) and date of registration should be included as the last line of the manuscript abstract.
For clinical trials that have not been registered prospectively, authors are encouraged to register retrospectively to ensure the complete publication of all results. The trial registration number (TRN), date of registration and the words ‘retrospectively registered’ should be included as the last line of the manuscript abstract.
Standards of reporting
SCI Index advocates complete and transparent reporting of biomedical and biological research and research with biological applications. Authors are recommended to adhere to the minimum reporting guidelines hosted by the EQUATOR Network when preparing their manuscript.
Exact requirements may vary depending on the journal; please refer to the journal’s Instructions for Authors.
Checklists are available for a number of study designs, including:
Randomised trials (CONSORT) and Study protocols (SPIRIT)
Observational studies (STROBE)
Systematic reviews and meta-analyses (PRISMA) and protocols (Prisma-P)
Diagnostic/prognostic studies (STARD) and (TRIPOD)
Case reports (CARE)
Clinical practice guidelines (AGREE) and (RIGHT)
Qualitative research (SRQR) and (COREQ)
Animal pre-clinical studies (ARRIVE)
Quality improvement studies (SQUIRE)
Economic evaluations (CHEERS)
Summary of requirements
The above should be summarized in a statement and placed in a ‘Declarations’ section before the reference list under a heading of ‘Ethics approval’.
Please see the various examples of wording below and revise/customize the sample statements according to your own needs.
Examples of statements to be used when ethics approval has been obtained:
Examples of statements to be used for a retrospective study:
Examples of statements to be used when no ethical approval is required/exemption granted:
Authors are responsible for correctness of the statements provided in the manuscript. See also Authorship Principles. The Editor-in-Chief reserves the right to reject submissions that do not meet the guidelines described in this section.
All individuals have individual rights that are not to be infringed. Individual participants in studies have, for example, the right to decide what happens to the (identifiable) personal data gathered, to what they have said during a study or an interview, as well as to any photograph that was taken. This is especially true concerning images of vulnerable people (e.g. minors, patients, refugees, etc) or the use of images in sensitive contexts. In many instances authors will need to secure written consent before including images.
Identifying details (names, dates of birth, identity numbers, biometrical characteristics (such as facial features, fingerprint, writing style, voice pattern, DNA or other distinguishing characteristic) and other information) of the participants that were studied should not be published in written descriptions, photographs, and genetic profiles unless the information is essential for scholarly purposes and the participant (or parent/guardian if the participant is a minor or incapable or legal representative) gave written informed consent for publication. Complete anonymity is difficult to achieve in some cases. Detailed descriptions of individual participants, whether of their whole bodies or of body sections, may lead to disclosure of their identity. Under certain circumstances consent is not required as long as information is anonymized and the submission does not include images that may identify the person.
Informed consent for publication should be obtained if there is any doubt. For example, masking the eye region in photographs of participants is inadequate protection of anonymity. If identifying characteristics are altered to protect anonymity, such as in genetic profiles, authors should provide assurance that alterations do not distort meaning.
Exceptions where it is not necessary to obtain consent:
Consent and already available data and/or biologic material
Regardless of whether material is collected from living or dead patients, they (family or guardian if the deceased has not made a pre-mortem decision) must have given prior written consent. The aspect of confidentiality as well as any wishes from the deceased should be respected.
Data protection, confidentiality and privacy
When biological material is donated for or data is generated as part of a research project authors should ensure, as part of the informed consent procedure, that the participants are made aware what kind of (personal) data will be processed, how it will be used and for what purpose. In case of data acquired via a biobank/biorepository, it is possible they apply a broad consent which allows research participants to consent to a broad range of uses of their data and samples which is regarded by research ethics committees as specific enough to be considered “informed”. However, authors should always check the specific biobank/biorepository policies or any other type of data provider policies (in case of non-bio research) to be sure that this is the case.
Consent to Participate
For all research involving human subjects, freely-given, informed consent to participate in the study must be obtained from participants (or their parent or legal guardian in the case of children under 16) and a statement to this effect should appear in the manuscript. In the case of articles describing human transplantation studies, authors must include a statement declaring that no organs/tissues were obtained from prisoners and must also name the institution(s)/clinic(s)/department(s) via which organs/tissues were obtained. For manuscripts reporting studies involving vulnerable groups where there is the potential for coercion or where consent may not have been fully informed, extra care will be taken by the editor and may be referred to the SCI Index Research Integrity Group.
Consent to Publish
Individuals may consent to participate in a study, but object to having their data published in a journal article. Authors should make sure to also seek consent from individuals to publish their data prior to submitting their paper to a journal. This is in particular applicable to case studies.
Summary of requirements
The above should be summarized in a statement and placed in a ‘Declarations’ section before the reference list under a heading of ‘Consent to participate’ and/or ‘Consent to publish’. Other declarations include Funding, Competing interests, Ethics approval, Consent, Data and/or Code availability and Authors’ contribution statements.
Please see the various examples of wording below and revise/customize the sample statements according to your own needs.
Sample statements for “Consent to participate”:
Informed consent was obtained from all individual participants included in the study.
Informed consent was obtained from legal guardians.
Written informed consent was obtained from the parents.
Verbal informed consent was obtained prior to the interview.
Sample statements for “Consent to publish”:
The authors affirm that human research participants provided informed consent for publication of the images in Figure(s) 1a, 1b and 1c.
The participant has consented to the submission of the case report to the journal.
Patients signed informed consent regarding publishing their data and photographs.
Sample statements if identifying information about participants is available in the article:
Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Authors are responsible for correctness of the statements provided in the manuscript. See also Authorship Principles. The Editor-in-Chief reserves the right to reject submissions that do not meet the guidelines described in this section.
Images will be removed from publication if authors have not obtained informed consent or the paper may be removed and replaced with a notice explaining the reason for removal.
Open Choice allows you to publish open access in more than 1850 SCI Index journals, making your research more visible and accessible immediately on publication.
Benefits:
It is easy to find funding to support open access – please see our funding and support pages for more information.
*) Within the first three years of publication.SCI Index hybrid journal OA impact analysis, 2018.
Copyright
Open Choice articles do not require transfer of copyright as the copyright remains with the author. In opting for open access, the author(s) agree to publish the article under a Creative Commons license. Details of the OA licences offered to authors can be found on the individual journal website, in the journal’s How to publish with us guide.
How can you help improve your manuscript for publication?
Presenting your work in a well-structured manuscript and in well-written English gives it its best chance for editors and reviewers to understand it and evaluate it fairly. Many researchers find that getting some independent support helps them present their results in the best possible light. The experts at sciindex Nature Author Services can help you with manuscript preparation—including English language editing, developmental comments, manuscript formatting, figure preparation, translation, and more.
You can also use our free Grammar Check tool for an evaluation of your work.
Please note that using these tools, or any other service, is not a requirement for publication, nor does it imply or guarantee that editors will accept the article, or even select it for peer review.
您怎么做才有助于改进您的稿件以便顺利发表?
如果在结构精巧的稿件中用精心组织的英语展示您的作品,就能最大限度地让编辑和审稿人理解并公正评估您的作品。许多研究人员发现,获得一些独立支持有助于他们以尽可能美好的方式展示他们的成果。sciindex Nature Author Services 的专家可帮助您准备稿件,具体包括润色英语表述、添加有见地的注释、为稿件排版、设计图表、翻译等。
您还可以使用我们的免费语法检查工具来评估您的作品。
请注意,使用这些工具或任何其他服务不是发表前必须满足的要求,也不暗示或保证相关文章定会被编辑接受(甚至未必会被选送同行评审)。
発表に備えて、論文を改善するにはどうすればよいでしょうか?
内容が適切に組み立てられ、質の高い英語で書かれた論文を投稿すれば、編集者や査読者が論文を理解し、公正に評価するための最善の機会となります。多くの研究者は、個別のサポートを受けることで、研究結果を可能な限り最高の形で発表できると思っています。sciindex Nature Author Servicesのエキスパートが、英文の編集、建設的な提言、論文の書式、図の調整、翻訳など、論文の作成をサポートいたします。
原稿の評価に、無料の文法チェックツールもご利用いただけます。
これらのツールや他のサービスをご利用いただくことは、論文を掲載するための要件ではありません。また、編集者が論文を受理したり、査読に選定したりすることを示唆または保証するものではないことにご注意ください。
게재를 위해 원고를 개선하려면 어떻게 해야 할까요?
여러분의 작품을 체계적인 원고로 발표하는 것은 편집자와 심사자가 여러분의 연구를 이해하고 공정하게 평가할 수 있는 최선의 기회를 제공합니다. 많은 연구자들은 어느 정도 독립적인 지원을 받는 것이 가능한 한 최선의 방법으로 자신의 결과를 발표하는 데 도움이 된다고 합니다. sciindex Nature Author Services 전문가들은 영어 편집, 발전적인 논평, 원고 서식 지정, 그림 준비, 번역 등과 같은 원고 준비를 도와드릴 수 있습니다.
또한 당사의 무료 문법 검사도구를 사용하여 여러분의 연구를 평가할 수 있습니다.
이러한 도구 또는 기타 서비스를 사용하는 것은 게재를 위한 필수 요구사항이 아니며, 편집자가 해당 논문을 수락하거나 피어 리뷰에 해당 논문을 선택한다는 것을 암시하거나 보장하지는 않습니다.
Open access articles in SciIndex journals are published under Creative Commons licences. These provide an industry-standard framework to support easy re-use of open access material. Under Creative Commons licences, authors retain copyright of their articles.
Articles are published open access under a CC BY licence (Creative Commons Attribution 4.0 International licence). CC BY articles may be shared and adapted for any purpose, including commercially, so long as the authors are credited.
You may also wish to find out about licence variations that are available to meet funder and institutional open access licence requirements.
14 days
Time to first decision
160 days
Review time
223 days
Submission to acceptance
20 days
Acceptance to publication
aims & scope
The Global Journal of Social Development Studies (JSDS) focuses on three main areas of discussion: community empowerment, corporate social responsibility, and social policy. The journal welcomes papers that discuss the following themes: Social Movement and Empowerment; Human Rights, Citizenship and Development; Social Entrepreneurship; Community Development Theories, Approaches and Methods; Community Organization and Participation; Socio-cultural, Environmental and Economic Development; Industrial Relations; Decent Work; Education and Social Policy; Health Insurance and Policy; Social Protection; Wellbeing, Welfare, and Development; Poverty and Social Justice; Social Inclusion; Gender, Family and Development; Digital Technology and Development; Welfare Regime.